A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
NCERT EXEMPLAR-SOLID STATE-Solid State
- the number of tetrahedral voids per unit cell in NaCl crystal is ...
Text Solution
|
- Amorphous solids can also be callled ………….. .
Text Solution
|
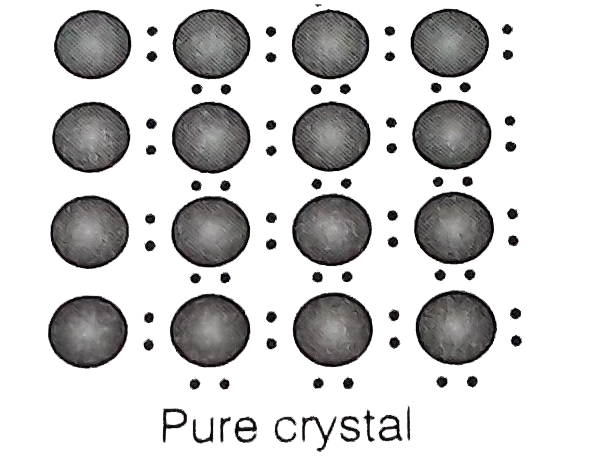
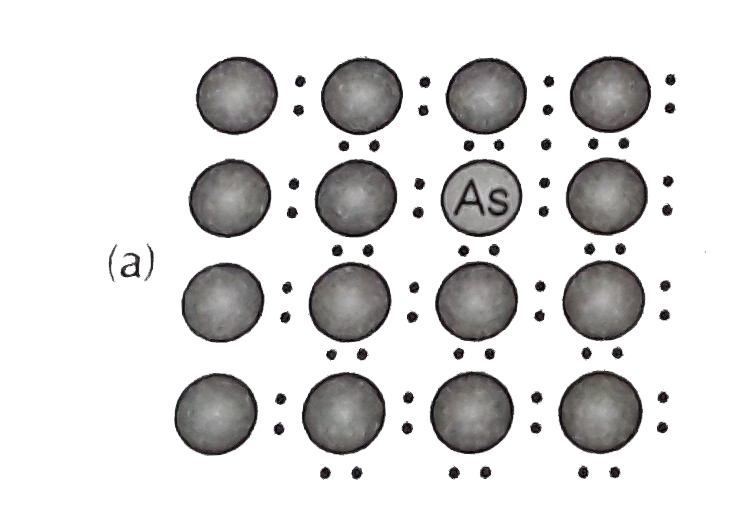
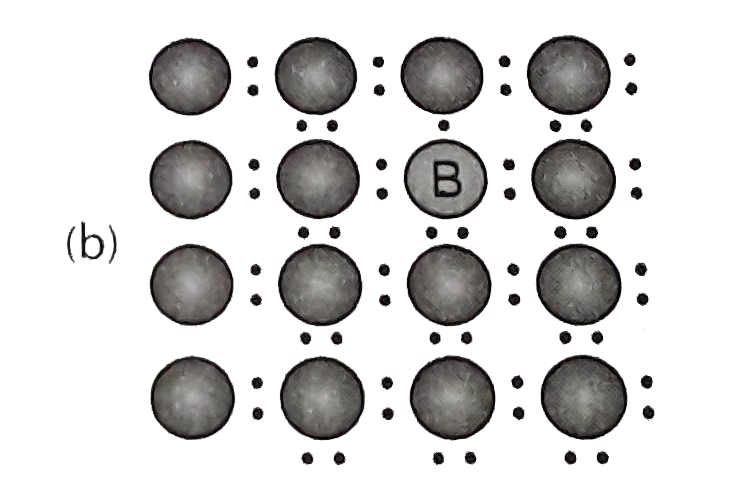
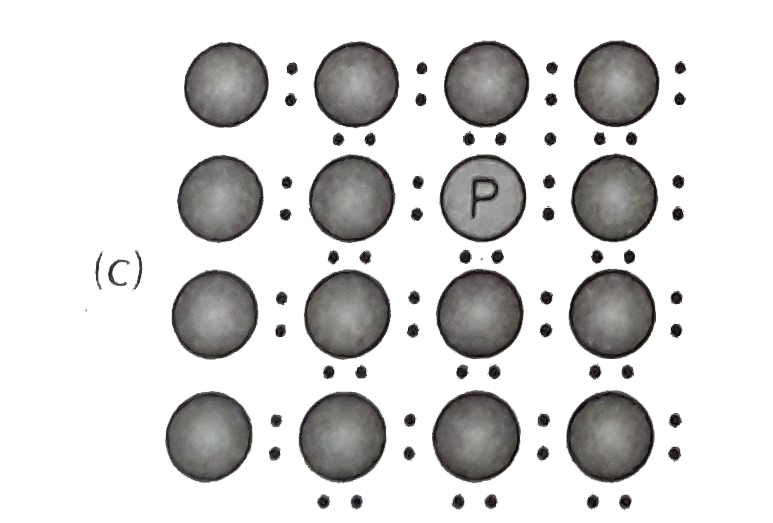
- A perfect crystal of silicon (fig) is deped with some elements a...
Text Solution
|
- which of the following statements are correct ?
Text Solution
|
- which of the following feastures are not shown by quartz glass ?
Text Solution
|
- which of the following cannot regarded as molecular solid ?
Text Solution
|
- in which of the following arrangement octahedral voids are formed...
Text Solution
|
- Frenkel defect is also known as ……… .
Text Solution
|
- which of the following defects decrase the density decrease the d...
Text Solution
|
- why are liquids and gases categorised as fuids ?
Text Solution
|
- why are solids incomressible ?
Text Solution
|
- Inspite of long range order in the arrangement of particles why...
Text Solution
|
- Why common salt (NaCl) sometimes appear yellow?
Text Solution
|
- why is Fe0(s) not formed in stoichiometric compostion ?
Text Solution
|
- why does white Zn0(s) becomes yellow upon heating ?
Text Solution
|
- why does the electrical conductivity of semiconductors increse w...
Text Solution
|
- Expalin why does conductivity of germainum crystals increase on ...
Text Solution
|
- A compound formed by two elements M and N. Element N forms ccp and ato...
Text Solution
|
- Under which situations can an amorphous substance change to cryst...
Text Solution
|
- match the defects given in column I with the statements in given...
Text Solution
|