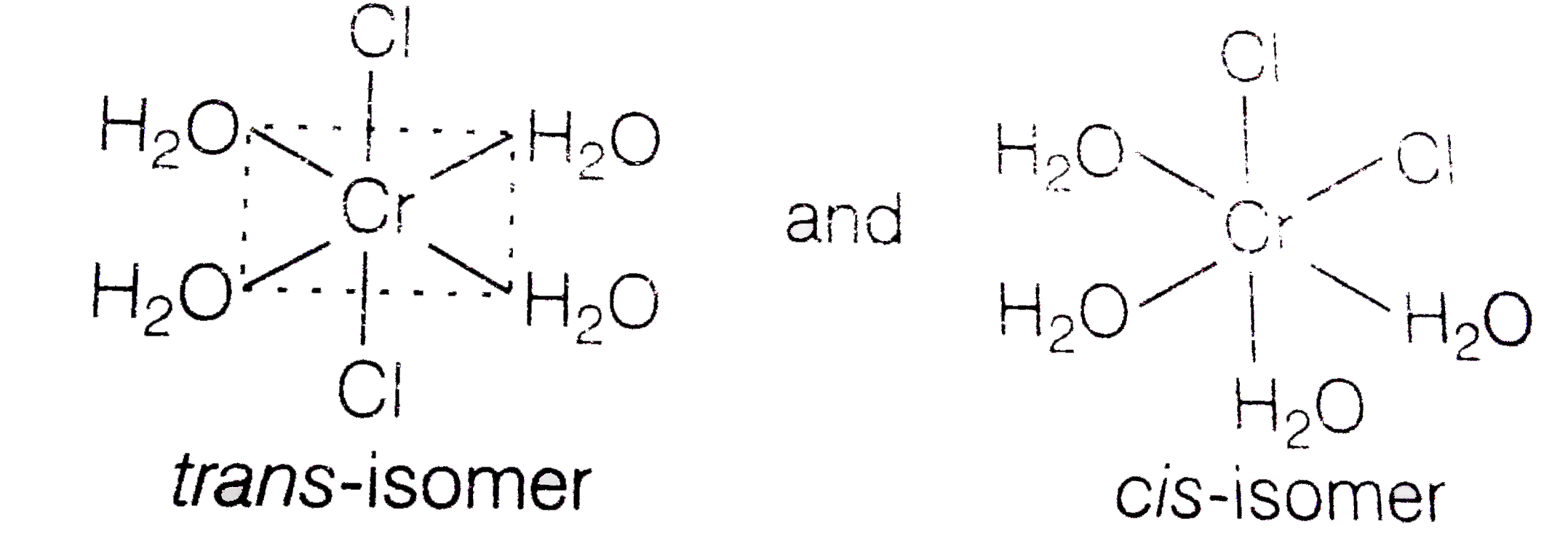A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
NCERT EXEMPLAR-COORDINATION COMPOUNDS-Coordination Compounds
- The correct IUPAC name of [Pt(NH(3))(2)Cl(2)] is
Text Solution
|
- The stabilization of coordination compound due to chelation is called ...
Text Solution
|
- Indicate the complex ion which shows geometrical isomerism.
Text Solution
|
- The CFSE for octahedral [CoCl(6)]^(4-) is 18,000 cm^(-1). The CFSE for...
Text Solution
|
- Due to the presence of ambidenate ligands coordination compounds show ...
Text Solution
|
- The two compounds [Co(SO(4))(NH(3))(5)]Br and [Co(SO(4))(NH(3))(5)]Cl ...
Text Solution
|
- Which of the following is not chelating agent (a) Thiosulphate (b)...
Text Solution
|
- Which of the following species is not expected to be a ligand?
Text Solution
|
- What kind of isomerism exists between [Cr(H(2)O)(6)]Cl(3) (violet) and...
Text Solution
|
- IUPAC name of [Pt(NH(3))(2)Cl(NO(2))] is
Text Solution
|
- Atomic number of Mn. Fe and Co are 25, 26 and 27 respectively. Which o...
Text Solution
|
- Atomic number of Mn, Fe, Co and Ni are 25, 26, 27 and 28 respectively....
Text Solution
|
- Which of the following options are correct for [Fe(CN)(6)]^(3-) comple...
Text Solution
|
- An aqueous pink solution of cobalt (II) chloride changes to deep blue ...
Text Solution
|
- Which of the following complexes are homoleptic ?
Text Solution
|
- Which of the following complexes are heteroleptic ?
Text Solution
|
- Identify the optically active compounds from the following
Text Solution
|
- Identify the correct statements for the behaviour of ethane-1, 2-diami...
Text Solution
|
- Which of the following complexes show linkage isomerism ?
Text Solution
|
- Arrange the following complexes in the increasing order of conductivit...
Text Solution
|