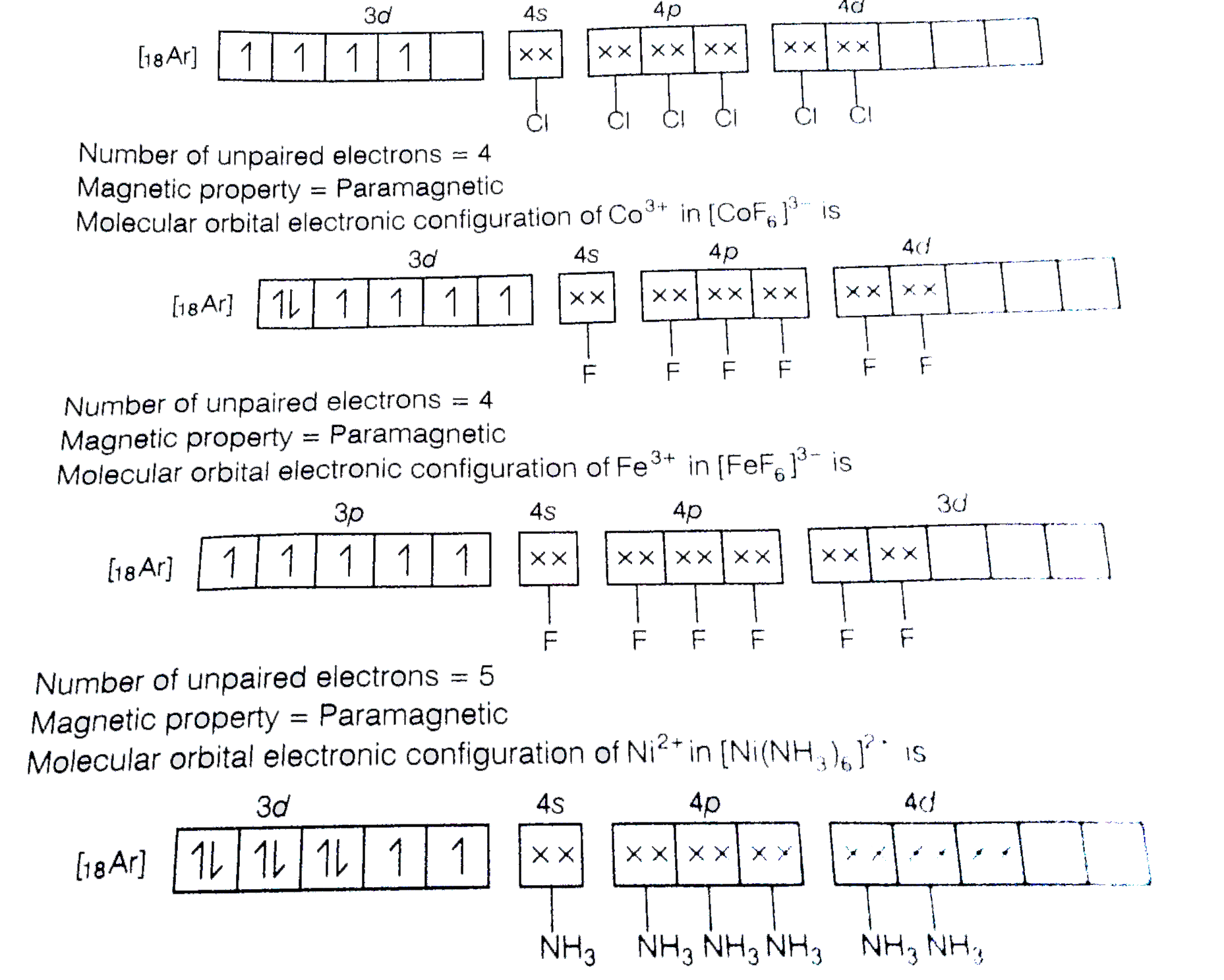A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
NCERT EXEMPLAR-COORDINATION COMPOUNDS-Coordination Compounds
- IUPAC name of [Pt(NH(3))(2)Cl(NO(2))] is
Text Solution
|
- Atomic number of Mn. Fe and Co are 25, 26 and 27 respectively. Which o...
Text Solution
|
- Atomic number of Mn, Fe, Co and Ni are 25, 26, 27 and 28 respectively....
Text Solution
|
- Which of the following options are correct for [Fe(CN)(6)]^(3-) comple...
Text Solution
|
- An aqueous pink solution of cobalt (II) chloride changes to deep blue ...
Text Solution
|
- Which of the following complexes are homoleptic ?
Text Solution
|
- Which of the following complexes are heteroleptic ?
Text Solution
|
- Identify the optically active compounds from the following
Text Solution
|
- Identify the correct statements for the behaviour of ethane-1, 2-diami...
Text Solution
|
- Which of the following complexes show linkage isomerism ?
Text Solution
|
- Arrange the following complexes in the increasing order of conductivit...
Text Solution
|
- A coordination compound CrCI(3).4H(2)O precipitates AgCI when treated ...
Text Solution
|
- A complex of the type [M(A A)(2)X(2)] is known to the optically active...
Text Solution
|
- Magnetic moment of [MnCl(4)]^(2-) is 5.92 BM. Explain giving reason pr...
Text Solution
|
- On the basis of crystal field theory explain why Co(III) Forms paramag...
Text Solution
|
- Why are low spin tetrahedral complexes not formed ?
Text Solution
|
- Give the electronic configuration of the following complexes on the ba...
Text Solution
|
- Explain why [Fe(H(2)O)(6)]^(3+) has magnetic moment value of 5.92 BM w...
Text Solution
|
- Arrange following complex ions in increasing order of crystal field sp...
Text Solution
|
- Why do compounds having similar geometry have different magnetic momen...
Text Solution
|