Similar Questions
Explore conceptually related problems
Recommended Questions
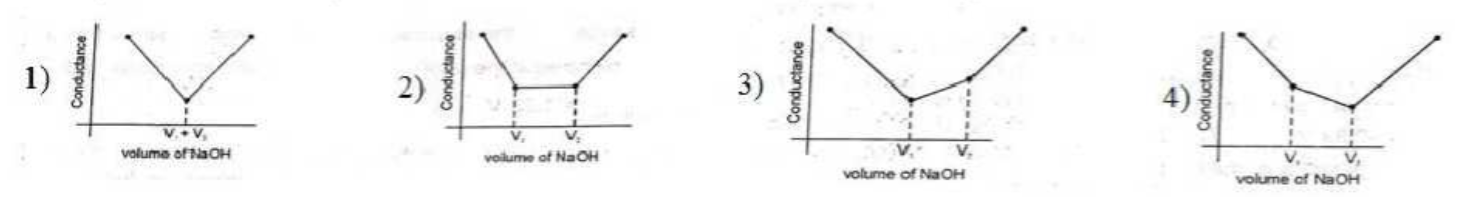
- The most appropriate titration curve obtained when a mixture of a stro...
Text Solution
|
- The best indicator for detection of end point in titration of a weak a...
Text Solution
|
- Which of the following statements about a weak acid strong base titrat...
Text Solution
|
- The best indicator for the detection of the end point in the titration...
Text Solution
|
- Which of the following curves corresponds to the titration of a weak b...
Text Solution
|
- (A) In general phenolphthalein is used as an indicator for the titrat...
Text Solution
|
- When a strong acid is titrated using a weak base, the pH at the equiva...
Text Solution
|
- दुर्लब अम्ल तथा प्रबल क्षारक के मध्य अनुमापन के लिए सावर्धिक उपयुक्त ...
Text Solution
|
- The most appropriate titration curve obtained when a mixture of a stro...
Text Solution
|