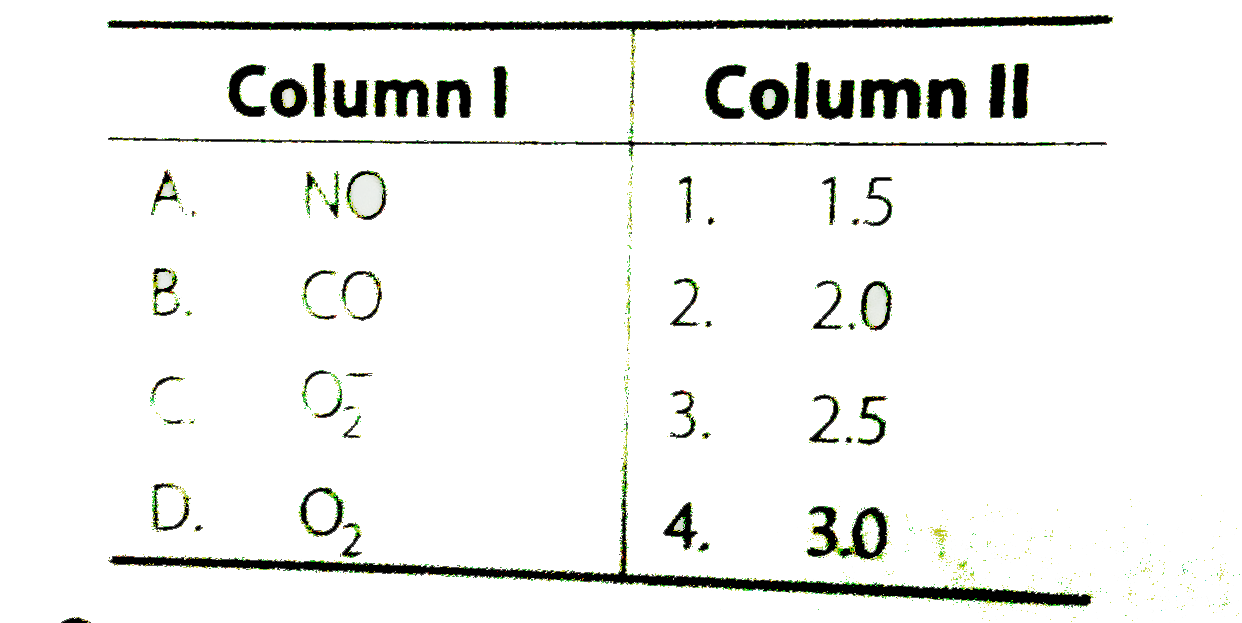Text Solution
Verified by Experts
Topper's Solved these Questions
CHEMICAL BONDING AND MOLECULAR STRUCTURE
NCERT EXEMPLAR|Exercise Assertions and Reasons|3 VideosCHEMICAL BONDING AND MOLECULAR STRUCTURE
NCERT EXEMPLAR|Exercise Long Answer type questions|9 VideosCHEMICAL BONDING AND MOLECULAR STRUCTURE
NCERT EXEMPLAR|Exercise Short answer types questions|21 VideosCLASSIFICATION OF ELEMENTS AND PERIODICITY IN PROPERTIES.
NCERT EXEMPLAR|Exercise Long answer types question|7 Videos
Similar Questions
Explore conceptually related problems
NCERT EXEMPLAR-CHEMICAL BONDING AND MOLECULAR STRUCTURE-Matching the columns
- Match the species in Column I with the type of hybrid orbitals in Colu...
Text Solution
|
- Match the species in Column I with the geometry/shape in Column II.
Text Solution
|
- Match the species in Column I with the bond order in Column II.
Text Solution
|
- Match the items given in column i with example given in Column II
Text Solution
|
- Match the shape of molecules in Column I with the type of hybridisatio...
Text Solution
|