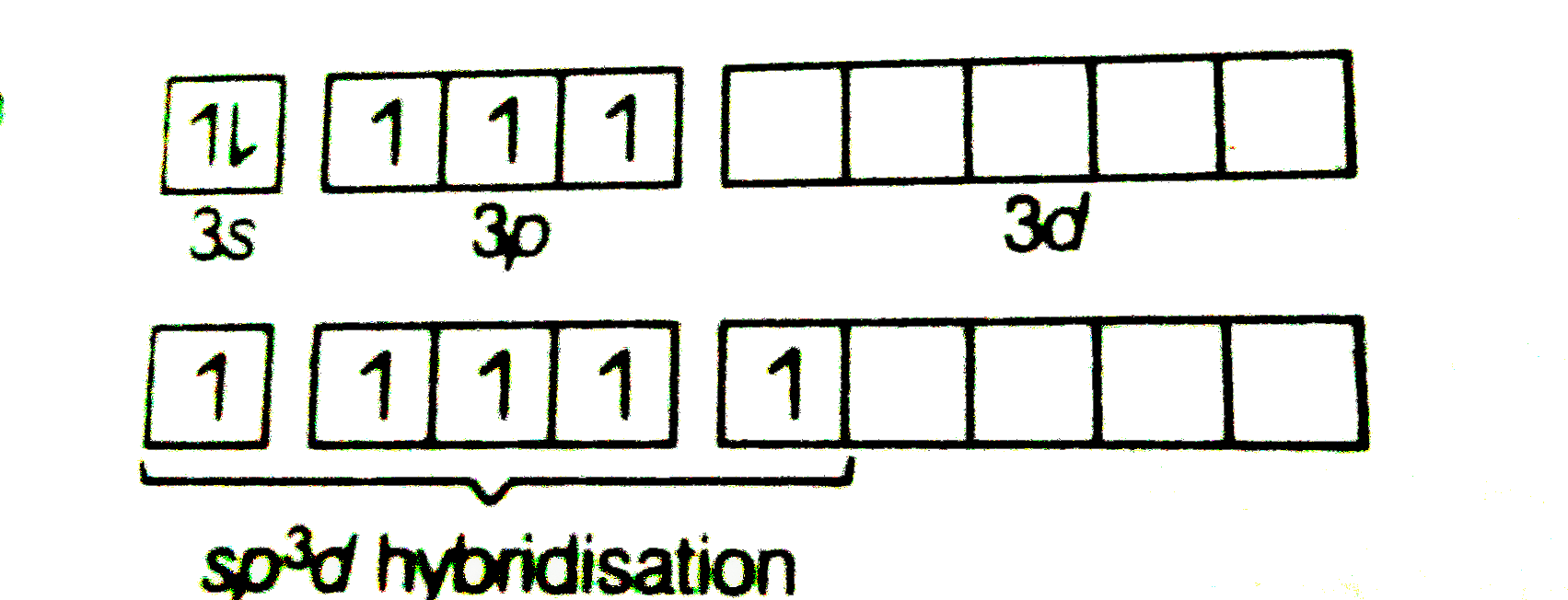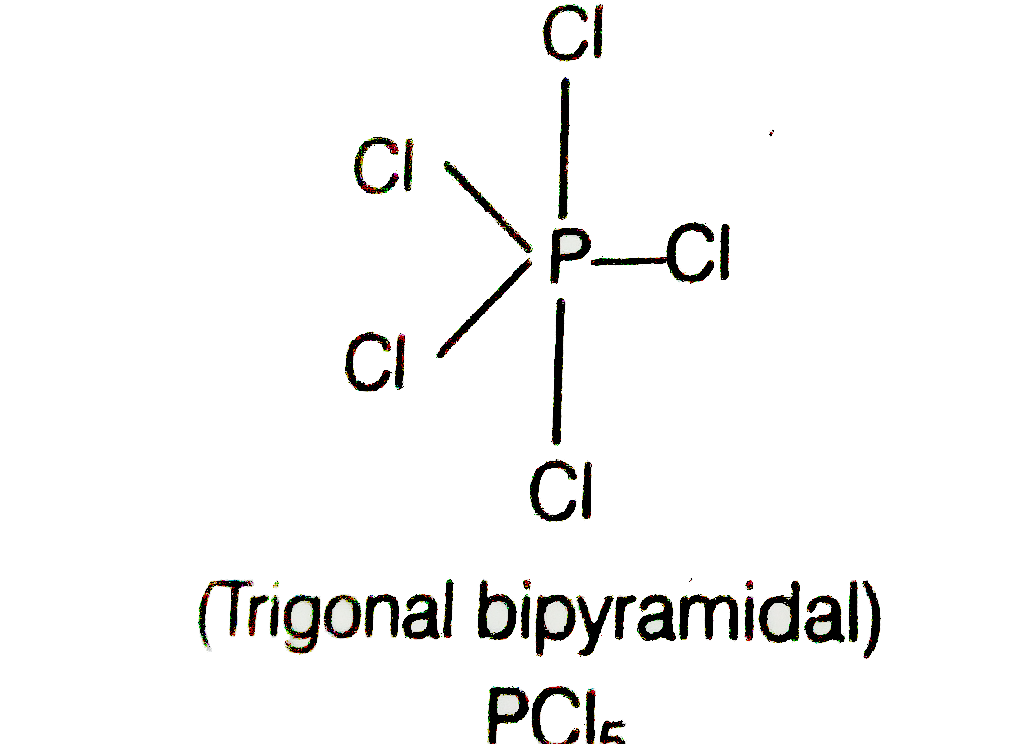Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
NCERT EXEMPLAR-CHEMICAL BONDING AND MOLECULAR STRUCTURE-Long Answer type questions
- a) Discuss the significance/applications of dipole moment. b) Repres...
Text Solution
|
- Use the molecular orbital energy level diagram to show that N(2) would...
Text Solution
|
- Briefly describe the valence bond theory of covalent bond formation by...
Text Solution
|
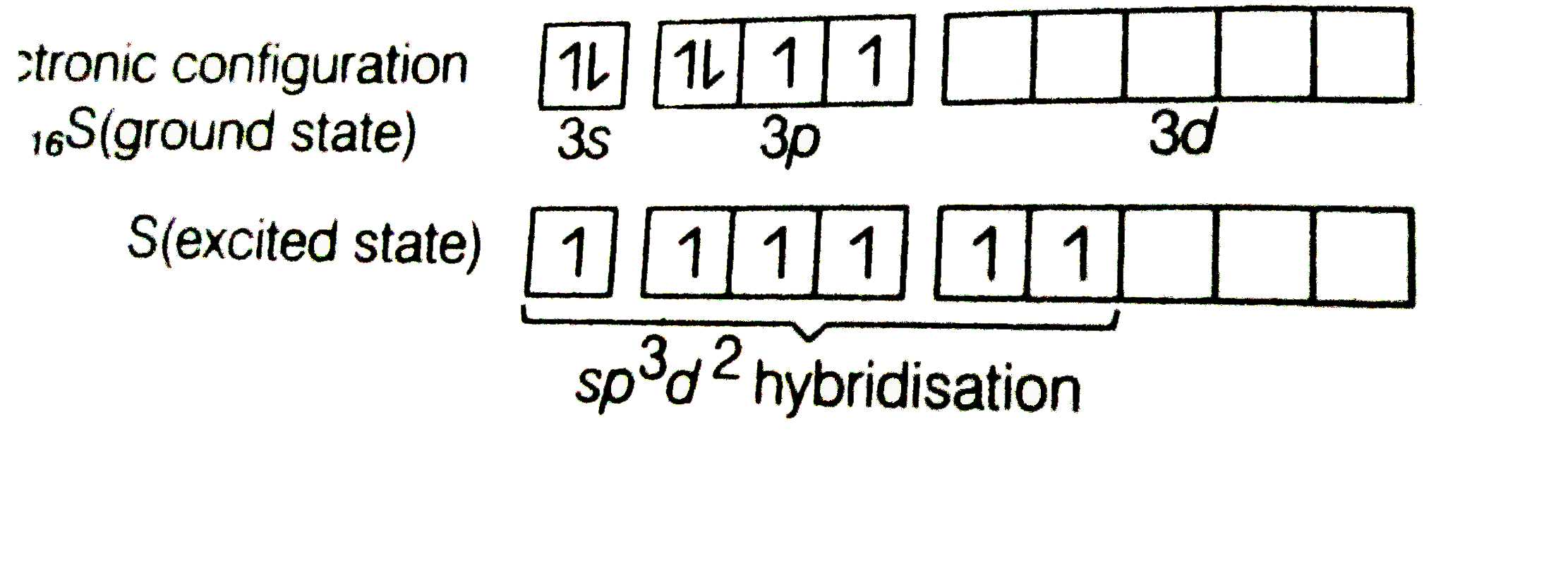
- Describe hybridisation in the case of PCl(5) and SF(5) The axial bonds...
Text Solution
|
- Discuss the concept of hybridisation. What are its different types in ...
Text Solution
|
- Comprehension given below is followed by some multiple choice question...
Text Solution
|
- Comprehension given below is followed by some multiple choice question...
Text Solution
|
- Comprehension given below is followed by some multiple choice question...
Text Solution
|
- Comprehension given below is followed by some multiple choice question...
Text Solution
|