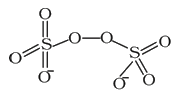
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- The ratio of oxygen atom having oxidation no. in S(2)O(B)^(2-)is :
Text Solution
|
- Statement Oxygen atom in both O(2) and O(3) has oxidation number zero....
Text Solution
|
- Assertion: Oxygen atom in both O(2) and O(3) has oxidation number zero...
Text Solution
|
- The two sulphur atoms in Na(2)S(2)O(3) have
Text Solution
|
- How many sulphur atoms in Na(2)S(4)O(6) have zero oxidation state?
Text Solution
|
- H(2)O(2) changes to O(2) What is the change in oxidation number of oxy...
Text Solution
|
- The order of increasing oxidation number of S in S(8),S(2),O(8)^(-2),S...
Text Solution
|
- S(4)O(6)^(2-) एवं S(2)O(8)^(2-) में S की ऑक्सीकरण संख्या है :
Text Solution
|
- Na(2)S(4)O(6) में S की ऑक्सीकरण संख्या होगी
Text Solution
|