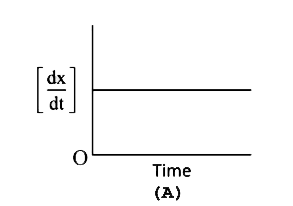
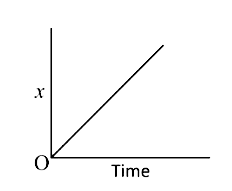
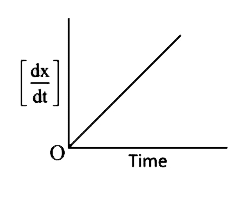
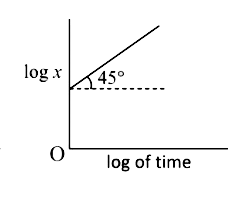
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- Which is not the graphical representation for the zeroth order reactio...
Text Solution
|
- How many half-lives are needed to complete the zeroth order reaction ?
Text Solution
|
- Graphical Representation
Text Solution
|
- Graphical Representation
Text Solution
|
- Graphical Representation
Text Solution
|
- Explain graphical representations of the first order reaction.
Text Solution
|
- Describe the graphical representation of first order reaction.
Text Solution
|
- If the initial concentration of the reactants is doubled in a zeroth o...
Text Solution
|
- With the help of a graphical representation explain how the rate of a ...
Text Solution
|