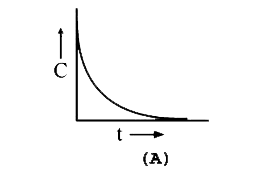
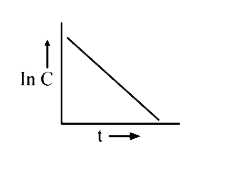
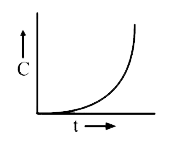
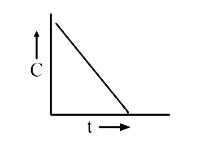
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- The plote between concentration versus time for a zero order reaction ...
Text Solution
|
- The plote between concentration versus time for a zero order reaction ...
Text Solution
|
- For a zero order reaction the plot of concentration of reactant versus...
Text Solution
|
- Which of the following graphs formed plotted between t(1//2) and initi...
Text Solution
|
- The plote between concentration versus time for a zero order reaction ...
Text Solution
|
- The plot between concentration versus time for a zero order reaction i...
Text Solution
|
- For a zero order reaction, the plot of concentration versus time is li...
Text Solution
|
- Which of the following graphs represent a first order reaction (a=init...
Text Solution
|
- शून्य कोटि की अभिक्रिया के लिए समय तथा सान्द्रता के मध्य ग्राफ खींचिए।
Text Solution
|