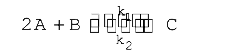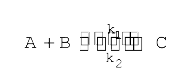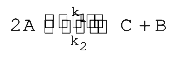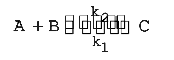A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- For a reaction of reversible nature, net rate is ((dx)/(dt))=k(1)[A][B...
Text Solution
|
- The rate and mechamical reaction are studied in chemical kinetics. The...
Text Solution
|
- The rate and mechamical reaction are studied in chemical kinetics. The...
Text Solution
|
- The rate and mechamical reaction are studied in chemical kinetics. The...
Text Solution
|
- The rate and mechamical reaction are studied in chemical kinetics. The...
Text Solution
|
- The rate and mechamical reaction are studied in chemical kinetics. The...
Text Solution
|
- The rate and mechamical reaction are studied in chemical kinetics. The...
Text Solution
|
- The rate and mechamical reaction are studied in chemical kinetics. The...
Text Solution
|
- For the reaction 2AtoB+3C, if -(d[A])/(dt)=k(1)[A]^(2),-(d[B])/(dt)=k...
Text Solution
|