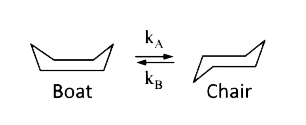A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- Consider the following reaction. The reaction is first order in each ...
Text Solution
|
- The energy of activation of a first order reaction is 187.06 kJ mol^(-...
Text Solution
|
- The rate constant for the decompoistion of a certain reaction is descr...
Text Solution
|
- The rate constant for the decompoistion of a certain reaction is descr...
Text Solution
|
- The time required for 10% completion of a first order reaction at 298 ...
Text Solution
|
- A graph plotted between log(10) K(c) and 1//T is straight line with in...
Text Solution
|
- The rate of chemical reaction are strongly affected by temperature . A...
Text Solution
|
- The rate of chemical reaction are strongly affected by temperature . A...
Text Solution
|
- Consider the following reaction. The reaction is first order in each ...
Text Solution
|