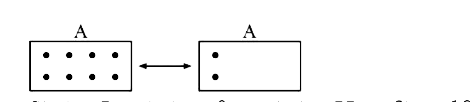
Text Solution
Verified by Experts
Similar Questions
Explore conceptually related problems
Recommended Questions
- If the rate law is -(d[A])/(dt) =k[A] State I, at t = 0 state I...
Text Solution
|
- For a reation: A rarr Product, rate law is -(d[A])/(dt)=K[A](0) . The ...
Text Solution
|
- The half life period of a first order reaction is 60 min. What percent...
Text Solution
|
- Half-life period of a first order reaction is 10 min. Starting with in...
Text Solution
|
- The rate law equation for the reaction A to B is found to be -(d[A])/(...
Text Solution
|
- The rate of change of concentration of (A) for reaction A to B is give...
Text Solution
|
- For a reaction A+Bto Products, rate law is -(d[A])/(dt)=k[A](0) . The ...
Text Solution
|
- For a first order reaction, half life is found to be 138.6 min, what w...
Text Solution
|
- An acid catalysed hydrolysis of ester follows rate law,R=k[CH3COOC2H5]...
Text Solution
|