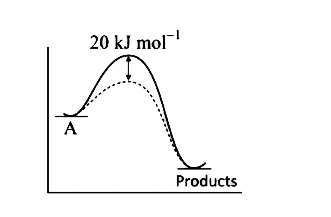
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- For the following reaction, A overset(500 K)rarr Products A overse...
Text Solution
|
- Mechanism of the reaction is: A(2) overset(k(2))hArr 2A A + B ove...
Text Solution
|
- A catalyst decreases E(a) form 100 KJ mol^(-1) to 80 KJ mol^(-1) At wh...
Text Solution
|
- MeC-=C-COCl overset(H(2)+ "Lindlar's Catalyst")(rarr)(A) The Product...
Text Solution
|
- Acetaldehyde overset("720 K")rarr The products of this reaction are
Text Solution
|
- Rate of reaction in absence of catalyst at 700 K is same as in presenc...
Text Solution
|
- A catalyst decreases activation energy by 15 Kcal and it is observed t...
Text Solution
|
- For following reactions A overset( 400K)( rarr) Product A underse...
Text Solution
|
- For the following reaction, A overset(500 K)rarr Products A overset(40...
Text Solution
|