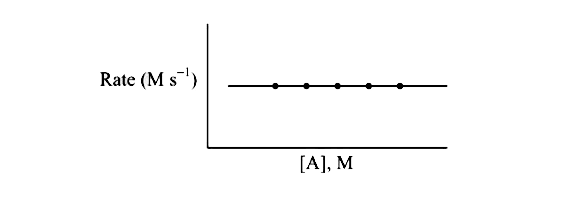
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- Following reaction can take place in both direction A {:(k(1)),(k(2)):...
Text Solution
|
- 2SO(2) + O(2) underset(k(2))overset(k(1))hArr 2SO(3) What are the e...
Text Solution
|
- The rate and mechamical reaction are studied in chemical kinetics. The...
Text Solution
|
- Following reaction can take place in both direction A overset(k(1))und...
Text Solution
|
- In a reaction A+BhArrC+D the rate constant of forward reaction & backw...
Text Solution
|
- In terms of rate constants for forward and backward reactions (k(f) an...
Text Solution
|
- For a reaction of reversible nature , net rate is ((dx)/(dt))=k(1)[A]^...
Text Solution
|
- K(1) and K(2) are the rate constants of forward and backward reactions...
Text Solution
|
- For a reaction of reversible nature, net rate is ((dx)/(dt))=k(1)[A][B...
Text Solution
|