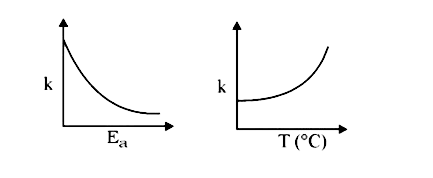
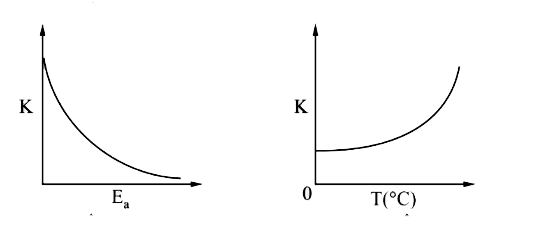
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- Consider the given plots for a reaction obeying Arrhenius equation (0^...
Text Solution
|
- Assertion (A) : k=Ae^(-E(a)//RT) , the Arrhenius equation represents t...
Text Solution
|
- Plots showing the variation of the rate constant (k) with temperature ...
Text Solution
|
- The temperature dependence of rate constant (k) of a chemical reaction...
Text Solution
|
- Consider the given plots for a reaction obeying Arrhenius equation (0^...
Text Solution
|
- The rate constant is given by Arrhenius equation. k=Ae^(-E(a)//RT) ...
Text Solution
|
- Plots showing the variation of the rate constant (k) with temperature ...
Text Solution
|
- Plots showing the variation of the rate constant (k) with temperature ...
Text Solution
|
- Plots showing the variation of the rate constant (k) with temperature ...
Text Solution
|