Text Solution
Verified by Experts
Similar Questions
Explore conceptually related problems
Recommended Questions
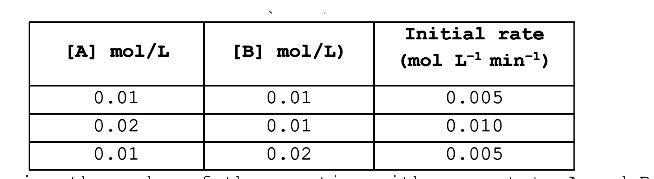
- Rate of reaction, A+B rarr products is given below as a function of di...
Text Solution
|
- Rate of a reaction A + B rarr Product, is given as a function of diffe...
Text Solution
|
- In a reaction between A and B, the initial rate of reaction was measur...
Text Solution
|
- The initial rates of reaction 3A + 2B + C to Products, at different ...
Text Solution
|
- The initial rate of reaction 3 A + 2 B + C to Products , at different ...
Text Solution
|
- In a reactio between A and B, the initial rate of reaction (r(0)) was ...
Text Solution
|
- For a reaction A + B rarr product, the rate of the reaction was double...
Text Solution
|
- Rate of reaction, A+B rarr products is given below as a function of di...
Text Solution
|
- In a reaction, between A and B, the initial rate of reaction (r(0)) wa...
Text Solution
|