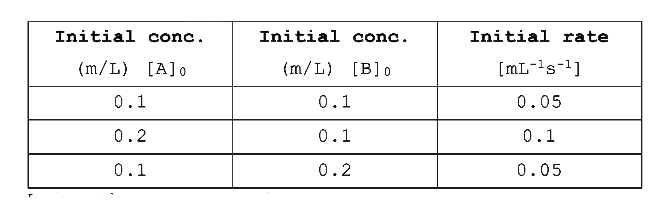Text Solution
Verified by Experts
Similar Questions
Explore conceptually related problems
Recommended Questions
- For the given reaction, A + B rarr Products Following data are given ...
Text Solution
|
- For the given reaction A+B rarr Products, the following data are g...
Text Solution
|
- For the given reaction, A+B rarr Products following data were obtaine...
Text Solution
|
- (a) For a reaction , A+B rarr Product the rate law is given rate = k[A...
Text Solution
|
- Rate of a reaction is given by the equation : Rate =k[A]^(2)[B] What a...
Text Solution
|
- For the given reaction, A + B rarr Products Following data are given (...
Text Solution
|
- Form the data given below for the reaction:A to B to Products the r...
Text Solution
|
- (a) For a reaction , A+B rarr Product the rate law is given rate = k[A...
Text Solution
|
- Rate of a reaction is given by the equation: Rate = k[A]^2[B]. What ar...
Text Solution
|