Text Solution
Verified by Experts
Similar Questions
Explore conceptually related problems
Recommended Questions
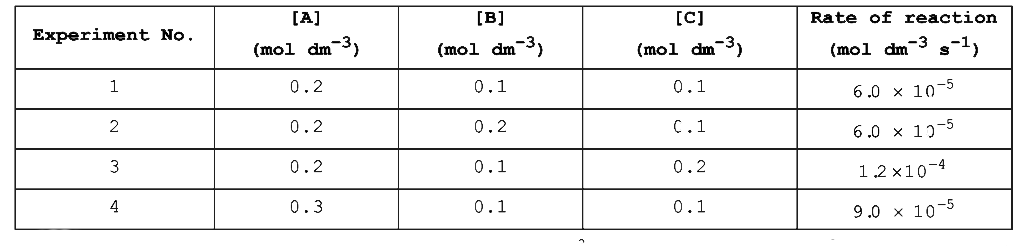
- Consider the kinetic data given in the following table for the reactio...
Text Solution
|
- In a first order reaction, the concentration of the reactant decreases...
Text Solution
|
- For a chemical reaction Ararr B ,the rate of the reaction is 2.0xx10^(...
Text Solution
|
- For a hypothetical reaction, the following kinetic data suggested that...
Text Solution
|
- In a reaction A+B hArr C+D , the initial concentrations of A and B wer...
Text Solution
|
- For a chemical reaction A to B , the rate of the reaction is 2 xx 10^(...
Text Solution
|
- The rate constant of a reaction is 10.8 xx 10^(-5) mol dm^(-3)s^(-1). ...
Text Solution
|
- The rate constant for reaction is 10.8 xx 10^(-5) mol dm^(-3) sec^(-1)...
Text Solution
|
- Consider the kinetic data given in the following table for the reactio...
Text Solution
|