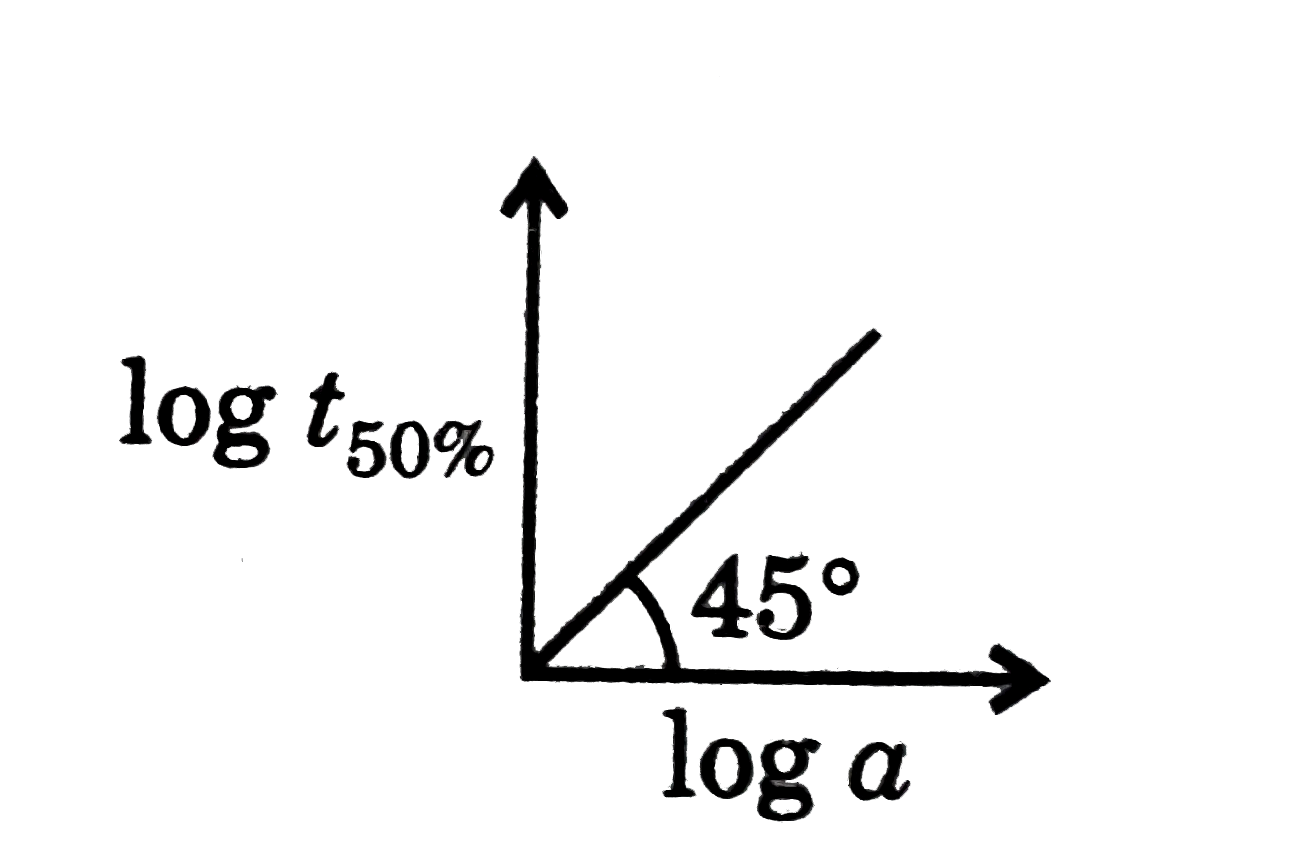A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Chemical Kinetics
MOTION|Exercise Exercise -1 (Experimental Method to calculate order)|5 VideosChemical Kinetics
MOTION|Exercise Exercise -1 (Methods to monitor the progress of reaction)|4 VideosChemical Kinetics
MOTION|Exercise Exercise -1 (Introduction, Rate of reaction, Factor affecting rate of reaction, Effect of concentration on reaction rate)|9 VideosCHEMICAL EQUILIBRIUM
MOTION|Exercise Exercise - 4|20 VideosCLASSROOM PROBLEMS
MOTION|Exercise Electrochemistry|22 Videos
Similar Questions
Explore conceptually related problems
MOTION-Chemical Kinetics-Exercise -1 (Zero order reaction, 1st order reaction , 2nd order reaction & nth order reaction)
- In a certain reaction, 10% of the reactant decomposes in one hour, 20%...
Text Solution
|
- The rate constant for the reaction A rarr B is 2xx10^(-4)"L mol"^(-1)"...
Text Solution
|
- What will be the order of reaction and rate constant for a chemical ch...
Text Solution
|
- For a first order reaction, the reaction, the plot of log C against 't...
Text Solution
|
- In presence of HCl, sucrose gets hydrolysed into glucose and fructose....
Text Solution
|
- In a first order of reaction the reacting substance has half-life peri...
Text Solution
|
- In the following first order reactions (A)overset(k(1))rarr"product",(...
Text Solution
|
- Two substances A (t(1//2)=5 min) and B(t(t//2)=10min) are taken in suc...
Text Solution
|
- If a I-order reaction is completed to the extent of 60% and 20% in tim...
Text Solution
|
- A drop of solution (volume 0.05 mL) contains 3 xx 10^(-6) "mole" H^(o+...
Text Solution
|
- The rate constant of reaction 2 A + B rarr C is 2.57 xx 10^(-5) L "mol...
Text Solution
|