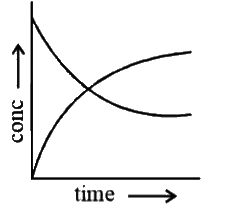
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Chemical Kinetics
MOTION|Exercise Exercise - 2 (Level-I) (Experimental Method to calculate order)|4 VideosChemical Kinetics
MOTION|Exercise Exercise - 2 (Level-I) (Arrhenius equation)|11 VideosChemical Kinetics
MOTION|Exercise Exercise - 2 (Level-I) (Introduction, Rate of reaction, Factor affecting rate of reaction, Effect of)|14 VideosCHEMICAL EQUILIBRIUM
MOTION|Exercise Exercise - 4|20 VideosCLASSROOM PROBLEMS
MOTION|Exercise Electrochemistry|22 Videos
Similar Questions
Explore conceptually related problems
MOTION-Chemical Kinetics-Exercise - 2 (Level-I) (Zero order reaction, 1st order reaction, 2nd order reaction & nth order reaction)
- Reaction A+BrarrC+D follows rate law .r=k[A]^(1//2)[B]^(1//2) Starting...
Text Solution
|
- Consider following two competing first ordr reactions, Poverset(k(1)...
Text Solution
|
- For a given reaction of first order, it takes 20 minutes for the conce...
Text Solution
|
- For a first order reaction, the concentration of reactant :
Text Solution
|
- Mathematical representation for t(1//4) life i.e., when 1//4th reactio...
Text Solution
|
- The decomposition of a gaseous substance (A) to yield gaseous products...
Text Solution
|
- The reaction A (g) rarr B(g) + 2C (g) is a first order reaction with r...
Text Solution
|
- For a reaction A rarr Products, the concentration of reactant are C(0)...
Text Solution
|
- A reaction 2A + B overset(k)rarr C+ D is first order with respect to A...
Text Solution
|
- For a certain reaction of order 'n' the time for half change t(1//2) i...
Text Solution
|
- Two first order reaction have half-life in the ratio 3:2. Calculate th...
Text Solution
|
- At the point of intersection of the two curves shown for the reaction ...
Text Solution
|