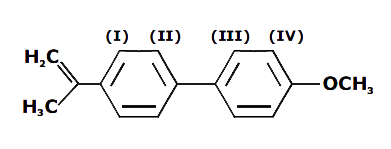A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
MOTION-AROMATIC COMPOUND-Exercise - 4 | Level-II
- Which position will be attacked most rapidly by the nitronium ion (NO(...
Text Solution
|
- Which of the following is obtained when 4- Methylbenzenesulphonic acid...
Text Solution
|
- <bbr> Phenol P and Q are respectively
Text Solution
|
- Reimer-Tiemann reaction introduces an aldehyde group on to the aromati...
Text Solution
|
- Reimer -Tiemann reaction introduces an aldehyde group on to the aromat...
Text Solution
|
- Reimer -Tiemann reaction introduces an aldehyde group on to the aromat...
Text Solution
|
- In the following reaction the structure of the major product ‘X’ ...
Text Solution
|
- Statement-I: p-Hydroxybenzoic acid has a lower boiling point that o-hy...
Text Solution
|
- Assertion: Bromobenzene upon reaction with Br(2)//Fe gives 1,4-dibromo...
Text Solution
|
- Statement I: Aniline on reaction with NaNO2HCl at 0^@C followed by co...
Text Solution
|
- The compounds P,Q and S were separately subjected to nitration us...
Text Solution
|
- In the reaction the intermediate (s) is (are)
Text Solution
|
- Amongst the compounds gives, the one that would form a brilliant color...
Text Solution
|
- In the following reaction, the product(s) formed is
Text Solution
|
- The the identification of beta-napthol using dye test, it is necessary...
Text Solution
|
- Among P,Q,R and S, the aromatic compound(s) is/ are
Text Solution
|
- The reaction of compounf P with CH(3)MgBr (excess) in (C(2)H(5)(2)O fo...
Text Solution
|