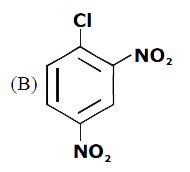
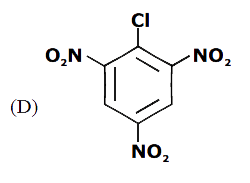
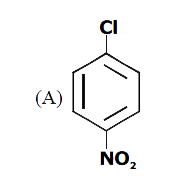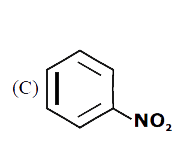A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
AROMATIC COMPOUND
MOTION|Exercise Exercise - 2 (Level-II)|29 VideosAROMATIC COMPOUND
MOTION|Exercise Exercise - 3|17 VideosAROMATIC COMPOUND
MOTION|Exercise Exercise - 4 | Level-II|16 VideosALKYI HALIDE
MOTION|Exercise Exercise - 4 | Level-II|8 VideosATOMIC STRUCTURE
MOTION|Exercise EXERCISE-4 (LEVEL-II)|18 Videos
Similar Questions
Explore conceptually related problems
MOTION-AROMATIC COMPOUND-Exercise - 2 (Level-I)
- The product is :
Text Solution
|
- C(6)H(6) +A overset(AlCl(3))(rarr) C(6)H(5)CONH(2) A in the above re...
Text Solution
|
- Which of the following is most reactive toward SNAr :
Text Solution
|
- Major, Product (B) is
Text Solution
|
- Reaction of SO(3) is faster in :
Text Solution
|
- The mixture of the following four aromatic compounds on oxidation by s...
Text Solution
|
- Methyl group attached to benzene can be oxidised to carboxyl group by ...
Text Solution
|
- Compound A and B respectively are :
Text Solution
|
- The number of benzylic hydrogen atoms in ethylbenzene is :
Text Solution
|
- The highest yield of m-product is possible by the electrophilic substi...
Text Solution
|
- Which of the following can be isolated as the product of this reaction...
Text Solution
|
- Which of the following is/are produced when a mixture of benzene vapou...
Text Solution
|
- Which of the following is the least reactive in the case of brominatio...
Text Solution
|
- Above compound undergoes
Text Solution
|
- Benzene on reaction with ‘A’ forms which on reaction with ‘B’ fo...
Text Solution
|
- In a reaction of C(6)H(5)Y the major product (gt60%) is m-isomer, so t...
Text Solution
|
- Which of the following will undergo sulphonation at fastest rate ?
Text Solution
|
- Which of the following is most reactive towards sulphonation ?
Text Solution
|
- When sulphonilic acid (p-H(2)NC(6)H(4)SO(3)H) is treated with excess o...
Text Solution
|
- Ring nitration of dimethyl benzene results in the formation of only on...
Text Solution
|