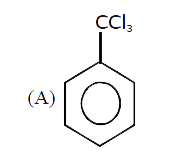
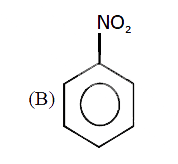
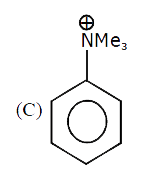
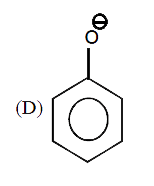
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
AROMATIC COMPOUND
MOTION|Exercise Exercise - 3|17 VideosAROMATIC COMPOUND
MOTION|Exercise Exercise - 4 | Level-I|13 VideosAROMATIC COMPOUND
MOTION|Exercise Exercise - 2 (Level-I)|47 VideosALKYI HALIDE
MOTION|Exercise Exercise - 4 | Level-II|8 VideosATOMIC STRUCTURE
MOTION|Exercise EXERCISE-4 (LEVEL-II)|18 Videos
Similar Questions
Explore conceptually related problems
MOTION-AROMATIC COMPOUND-Exercise - 2 (Level-II)
- Amongst the following, moderately activating groups is
Text Solution
|
- Which of the following is ortho para directing
Text Solution
|
- Of the species PhSH, Ph underset(O)underset("||")(S)R, ph underset(O)u...
Text Solution
|
- Which of the following group(s) activate the benzene ring towards elec...
Text Solution
|
- Statement-1 : Rate of nitration is C(6)H(6) cong C(6) D(6) cong C(6)T(...
Text Solution
|
- Product (A) will be
Text Solution
|
- Product (A) is
Text Solution
|
- Which aromatic compound is obtained when noctane undergoes catalytic h...
Text Solution
|
- Benzoic acid may be prepared by the oxidation of:
Text Solution
|
- Isopropylbenzene can be prepared by :
Text Solution
|
- In which of the following reaction t-butylbenzene is formed:
Text Solution
|
- Product is
Text Solution
|
- Which of the following reactions of bezene proves the presence of thre...
Text Solution
|
- Electrophile NO(2)^(o+) attacks the following in which cases NO(2)^(o...
Text Solution
|
- The reaction of replacement of a hydrogen atom in benzene by alkyl gro...
Text Solution
|
- Which of the following statements is correct:
Text Solution
|
- Which of the following gives Friedel Crafts reac- tion?
Text Solution
|
- Which of the following can be used in Friedel Crafts reaction when ben...
Text Solution
|
- The good method for converting benzene into n- propyl benzene is:
Text Solution
|
- Which of the following will undergo nitration slower than benzene ?
Text Solution
|