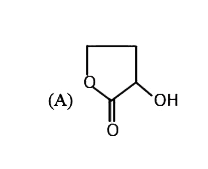
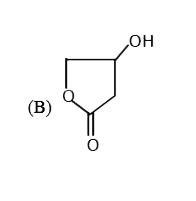
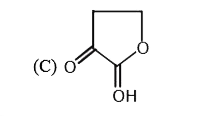
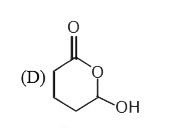
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
CARBONL COMPOUNDS
MOTION|Exercise EXERCISE -2 (LEVEL-II)|18 VideosCARBONL COMPOUNDS
MOTION|Exercise EXERCISE - 3|10 VideosCARBONL COMPOUNDS
MOTION|Exercise EXERCISE -1|40 VideosBIOMOLECULES & POLYMERS
MOTION|Exercise EXERCISE - 4 (LEVEL II)|17 VideosCARBOXYLIC ACID
MOTION|Exercise Exercise - 4 (LEVEL - 2 )|22 Videos
Similar Questions
Explore conceptually related problems
MOTION-CARBONL COMPOUNDS-EXERCISE - 2 (LEVEL -I)
- Which of the following compounds does not react with NaHSO(3)?
Text Solution
|
- Ph -CH = O + NH(2) - NH(2) overset(H^(oplus))(rarr) (A).Product (A)...
Text Solution
|
- HO-CH(2)-CH(2)-CH =O underset(underset((4)Delta)underset((3)H(2)O^(opl...
Text Solution
|
- Complete the following reaction
Text Solution
|
- (x)underset(H(2)O) overset(OH^(-))(rarr)beta -hydroxy ketone (x) can...
Text Solution
|
- which of the following product form when dil NaOH react
Text Solution
|
- major product structure of (x) is
Text Solution
|
- Aldol addition can be
Text Solution
|
- Structure of (x) is
Text Solution
|
- HCHO overset(Conc.NaOH)(rarr) (x) + (y) (i) Oxidation number of carb...
Text Solution
|
- Compound which gives cannizaro reaction?
Text Solution
|
- Compound which gives intramolecular aldol reaction?
Text Solution
|
- Degree of unsaturation in product is?
Text Solution
|
- This is an example of an intramolecular aldol Product (A) is:
Text Solution
|
- underset(Zn)overset(O(3)(1"mole"))(rarr) "(A)" overset(Conc.KOH)(rarr)...
Text Solution
|
- Product (B) is
Text Solution
|
- Product (B) is
Text Solution
|
- Complete the following reaction
Text Solution
|
- D - overset(O)overset(||)(C )-D overset(50%KOH)(rarr) order of the rea...
Text Solution
|
- In the reaction X + Y underset(5^(@)C)overset(NaOH)(rarr) CH(3)-ov...
Text Solution
|