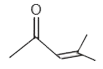
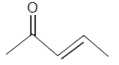
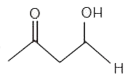
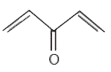
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
CARBONL COMPOUNDS
MOTION|Exercise EXERCISE -2 (LEVEL-II)|18 VideosCARBONL COMPOUNDS
MOTION|Exercise EXERCISE - 3|10 VideosCARBONL COMPOUNDS
MOTION|Exercise EXERCISE -1|40 VideosBIOMOLECULES & POLYMERS
MOTION|Exercise EXERCISE - 4 (LEVEL II)|17 VideosCARBOXYLIC ACID
MOTION|Exercise Exercise - 4 (LEVEL - 2 )|22 Videos
Similar Questions
Explore conceptually related problems
MOTION-CARBONL COMPOUNDS-EXERCISE - 2 (LEVEL -I)
- In the reaction X + Y underset(5^(@)C)overset(NaOH)(rarr) CH(3)-ov...
Text Solution
|
- Total number of products in the given reaction : (excluding steroisome...
Text Solution
|
- In the reaction :
Text Solution
|
- Product of Perkin reaction is :
Text Solution
|
- Cross cannizzaro reaction is example of
Text Solution
|
- Compound formed by the reaction of furfural with ethanol is
Text Solution
|
- underset(Zn)overset(O(3))(rarr) (A) underset(Delta)overset(KOH)(rarr) ...
Text Solution
|
- CH(3) - overset(O)overset(||)(C ) - CH(3) + H - overset(O)overset(||)(...
Text Solution
|
- CH(3) - overset(O)overset(||)(C ) - OH underset(Delta)overset(CaO)(rar...
Text Solution
|
- underset((ii)H(3)O^(oplus))overset((i)KCN)(rarr) P Find out the inco...
Text Solution
|
- Reagent (x) is
Text Solution
|
- Which of the following will reacts with NaOI ?
Text Solution
|
- underset(CH(2)OH)underset(|)overset(CHO)overset(|)((CHOH)(4)) rarr und...
Text Solution
|
- Which acid can be oxidised by Fehling solution:
Text Solution
|
- underset(KH "excess")overset("MeI excess")(rarr)81% yield, Product of ...
Text Solution
|
- Schiff's base is prepared from:
Text Solution
|
- Schiff’s reagent is used for the differentiation between:
Text Solution
|
- In the reaction sequence[X] is ketone: [X] overset("KMnO"4//overset(...
Text Solution
|
- Which will be give silver mirror test with Tollen's reagent?
Text Solution
|
- Acetaldehyde cannot give:
Text Solution
|