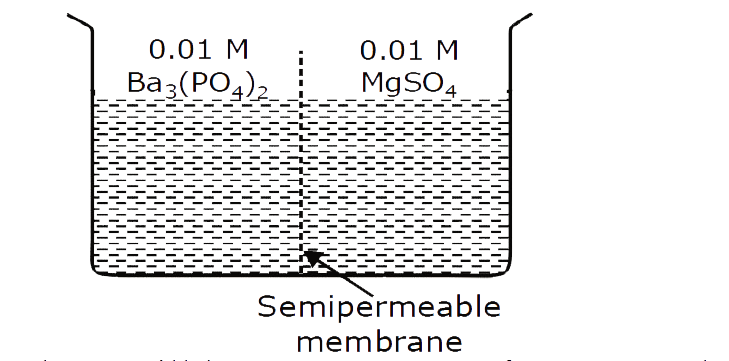A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
LIQUID SOLUTION
MOTION|Exercise EXERCISE-3 SUBJECTIVE JEE ADVANCED|40 VideosLIQUID SOLUTION
MOTION|Exercise EXERCISE-4 (Level-I) PREVIOUS YEAR JEE MAIN|30 VideosLIQUID SOLUTION
MOTION|Exercise EXERCISE-2 (LEVEL -I) OBJECTIVE PROBLEMS JEE MAIN|53 VideosISOMERISM
MOTION|Exercise Exercise 4|26 VideosMETALLURGY
MOTION|Exercise EXERCISE 4 (LEVEL - II)|17 Videos
Similar Questions
Explore conceptually related problems
MOTION-LIQUID SOLUTION-EXERCISE-2 (LEVEL -II) MULTIPLE CORRECT JEE ADVANCED
- Study the following figure and choose the correct options from the giv...
Text Solution
|
- When CuSO4 is dissolved in NH4OH solution then the correct statement i...
Text Solution
|
- The example of negative deviationis
Text Solution
|
- acetone and carbon disulphide form binary liquid solution showing posi...
Text Solution
|
- The vapour pressure of benzene and its solution with a non-electrolyte...
Text Solution
|
- A mixture of two volatile liquids A and B for 1 and 3 moles respectivl...
Text Solution
|
- What weight of solute (mol. Wt. 60) is required to dissolve in 180 g o...
Text Solution
|
- Statement Addition of a non-volatile solute causes a depression in ...
Text Solution
|
- Statement-I: 0.02m solutions of urea and sucrose will freeze at same t...
Text Solution
|
- Statement - I. For isotonic solutions C1 = C2 Statement - II. For i...
Text Solution
|
- Statement - I. Benzene-toluene mixture forms ideal solution. Statem...
Text Solution
|
- Statement - I. Dissolution of sulphuric acid in water gives a solution...
Text Solution
|
- Statement - I. Greater the molal depression constant of the solvent us...
Text Solution
|
- Statement - van't Hoff factor for electrolytes is always greater tha...
Text Solution
|
- Statement - The vapour pressure of 0.1 M sugar solution is more tha...
Text Solution
|
- Statement - I. Blood cells are isotonic with 0.16 M NaCl solution. ...
Text Solution
|
- Statement - I. Molarity of the solution changes with temperature. S...
Text Solution
|
- Statement-1 : The freezing of water is an endothermic process. State...
Text Solution
|
- Statement-1 : Addition of ethylene glycol (non-volatile) to water lowe...
Text Solution
|
- Column I (A) Ideal solution (B) Solutions showing positive deviati...
Text Solution
|