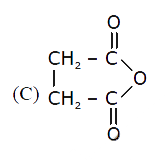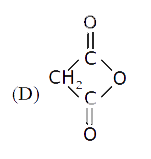A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
CARBOXYLIC ACID
MOTION|Exercise Exercise - 2(LEVEL - 2)|65 VideosCARBOXYLIC ACID
MOTION|Exercise Exercise - 3|16 VideosCARBOXYLIC ACID
MOTION|Exercise Exercise - 4 (LEVEL - 2 )|22 VideosCARBONL COMPOUNDS
MOTION|Exercise EXERCISE - 4 LEVEL -II|24 VideosCHEMICAL BONDING
MOTION|Exercise EXERCISE -4 LEVEL-II|40 Videos
Similar Questions
Explore conceptually related problems
MOTION-CARBOXYLIC ACID-Exercise - 2(LEVEL - 1)
Text Solution
|
- The product P of the following reaction is CH(3)-COOHoverset(LiAlH(4...
Text Solution
|
- Identify X in the reaction sequence Xunderset(C Cl(4))overset(Br(2))...
Text Solution
|
- The final product of the following reaction sequence is
Text Solution
|
- In the reaction sequence X & Y are
Text Solution
|
- In the above reaction sequence X & Y are.
Text Solution
|
- A racemic mixture of carboxylic acid having one chiral centre is treat...
Text Solution
|
- The product P of the following reaction is CH(3)-COOHoverset(LiAlH(4...
Text Solution
|
- Number of molecules of CO(2) which is produced on heating?
Text Solution
|
- In the above reaction Q is
Text Solution
|
- End product of this reaction is CH(3)-overset(O)overset(||)C-CH(2)-C...
Text Solution
|
- For the following conversion the correct sequence of reacgent is
Text Solution
|
Text Solution
|
- In the given reaction CH(3)COOH underset({:((ii)NaCN),((iii)H(2)O //...
Text Solution
|
- In the reaction sequence CH(3)-CH(2)-COOH overset(Br(2)//PBr(3))tox ...
Text Solution
|
Text Solution
|
Text Solution
|
- The correct sequence of reagent for the following conversion.
Text Solution
|
- CH(3)-underset((d))overset(C(2)H(5))overset(|)(CH)-COOH+CH(3)-underset...
Text Solution
|
- For the following acids the rate of decarboxylation on heating would b...
Text Solution
|