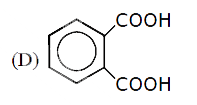
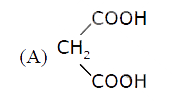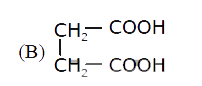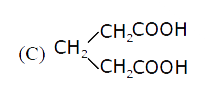A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
CARBOXYLIC ACID
MOTION|Exercise Exercise - 3|16 VideosCARBOXYLIC ACID
MOTION|Exercise Exercise - 4 (LEVEL - 1 )|15 VideosCARBOXYLIC ACID
MOTION|Exercise Exercise - 2(LEVEL - 1)|26 VideosCARBONL COMPOUNDS
MOTION|Exercise EXERCISE - 4 LEVEL -II|24 VideosCHEMICAL BONDING
MOTION|Exercise EXERCISE -4 LEVEL-II|40 Videos
Similar Questions
Explore conceptually related problems
MOTION-CARBOXYLIC ACID-Exercise - 2(LEVEL - 2)
Text Solution
|
- In the given reaction sequence (A) and (B) respectively are :
Text Solution
|
- Following will not yield a cyclic compound on heating :
Text Solution
|
- In the following reaction X overset(Delta)to Y. X is the lowest molecu...
Text Solution
|
- X overset(NH(3)//Delta)toB underset(Delta)overset(KOBr)to overset(HNO(...
Text Solution
|
- PhCH(2)-overset(CH(3))overset(|)(CH)-CH(2)-overset(O)overset(||)C-NH(2...
Text Solution
|
- Product X & Y are :–
Text Solution
|
- Urea (H(2)NCONH(2))+ hot dilute NaOH to X+NH(3), X is
Text Solution
|
Text Solution
|
Text Solution
|
- The increasing order of basicity of RCN,RCH=NR and RNH(2) is :-
Text Solution
|
- How many isomeric amines with formula C(7)H(9)N contain a benzene ring...
Text Solution
|
- in which of the following reaction, Amine is obtained as a product.
Text Solution
|
- Reaction of RCONH(2) with a mixture of Br(2) and KOH gives RNH(2) as t...
Text Solution
|
- Amines are highly soluble in
Text Solution
|
- Which of the following reagents can be used to convert benzenediazoniu...
Text Solution
|
- The bromination of aniline in presence of water produces :
Text Solution
|
- The compound which on rection with aqueous nirous acid at low temperat...
Text Solution
|
- Carbylamine test is performed in alcoholic KOH by heating a mixture of...
Text Solution
|
- Activation of benzene by -NH2 group can be reduced by treating the com...
Text Solution
|