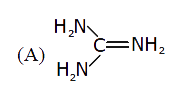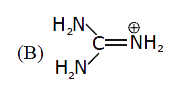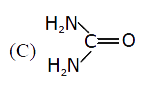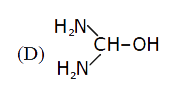A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
CARBOXYLIC ACID
MOTION|Exercise Exercise - 3|16 VideosCARBOXYLIC ACID
MOTION|Exercise Exercise - 4 (LEVEL - 1 )|15 VideosCARBOXYLIC ACID
MOTION|Exercise Exercise - 2(LEVEL - 1)|26 VideosCARBONL COMPOUNDS
MOTION|Exercise EXERCISE - 4 LEVEL -II|24 VideosCHEMICAL BONDING
MOTION|Exercise EXERCISE -4 LEVEL-II|40 Videos
Similar Questions
Explore conceptually related problems
MOTION-CARBOXYLIC ACID-Exercise - 2(LEVEL - 2)
- Which of the following reagents can be used to convert benzenediazoniu...
Text Solution
|
- The bromination of aniline in presence of water produces :
Text Solution
|
- The compound which on rection with aqueous nirous acid at low temperat...
Text Solution
|
- Carbylamine test is performed in alcoholic KOH by heating a mixture of...
Text Solution
|
- Activation of benzene by -NH2 group can be reduced by treating the com...
Text Solution
|
- Dipolar ion structure for amino acid is
Text Solution
|
- -NH(2) group shows acidic nature while reacts with reagent.
Text Solution
|
- Which of the following does not give ethylamine on reduction
Text Solution
|
- CH3 NH2 overset( "excess " CH3 Cl) to (X ) underset( "moist ") ...
Text Solution
|
- The product not obtained in the following reaction , CH (3) - NO2...
Text Solution
|
- Which of the following amines from N- nitroso derivative when treated ...
Text Solution
|
- The strongest base among the following is
Text Solution
|
- Identfiy compound (A) in the following oxidation reaction.
Text Solution
|
- p-Cl-C(6)H(4)NH(2) and PhNH(3)^(+)Cl^(-) can be distinguished by
Text Solution
|
- Acetamide is treated Separately with the following reagents. Which one...
Text Solution
|
- Treatment of ammonia with excess of ethyl chloride will yield
Text Solution
|
- C(6)H(5)C-=N and C(6)H(5)N-=C exhibit which type of isomerism
Text Solution
|
- The correct reagent sequence for the following conversion CH(3)-unde...
Text Solution
|
- Correct water solubility order/is amongst the following pairs is/are.
Text Solution
|
- The correct order/s for the given pair of isomers is /are
Text Solution
|