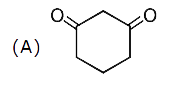
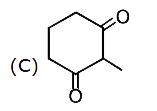
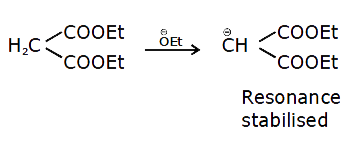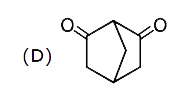A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
CARBOXYLIC ACID
MOTION|Exercise Exercise - 4 (LEVEL - 1 )|15 VideosCARBOXYLIC ACID
MOTION|Exercise Exercise - 4 (LEVEL - 2 )|22 VideosCARBOXYLIC ACID
MOTION|Exercise Exercise - 2(LEVEL - 2)|65 VideosCARBONL COMPOUNDS
MOTION|Exercise EXERCISE - 4 LEVEL -II|24 VideosCHEMICAL BONDING
MOTION|Exercise EXERCISE -4 LEVEL-II|40 Videos
Similar Questions
Explore conceptually related problems
MOTION-CARBOXYLIC ACID-Exercise - 3
- In organic chemistry various reactions take place by rearrangements. T...
Text Solution
|
- In organic chemistry various reactions take place by rearrangements. T...
Text Solution
|
- In organic chemistry various reactions take place by rearrangements. T...
Text Solution
|
- The conversion of an amide into an amine with one carbon atom less by ...
Text Solution
|
- The conversion of an amide into an amine with one carbon atom less by ...
Text Solution
|
- The conversion of an amide into an amine with one carbon atom less by ...
Text Solution
|
- Active methylene compounds are of great importance in synthetic chemis...
Text Solution
|
- Active methylene compounds are of great importance in synthetic chemis...
Text Solution
|
- Active methylene compounds are of great importance in synthetic chemis...
Text Solution
|
- Match the columns. {:(" Column-I","Column-II"),(" (Reaction)"...
Text Solution
|
- Match the columns. {:("Column-I (Pair)"," Column-II"),((a) CH(3)-...
Text Solution
|
- Match the Column-I with Column-II. {:("Column-I","Column-II"),((a)CH...
Text Solution
|
- Identify the intermediate product in the following conversion.
Text Solution
|
- Match the column. {:("Column-I","Column-I"),("(a) Schimdt reaction",...
Text Solution
|
- Find the product.
Text Solution
|
Text Solution
|