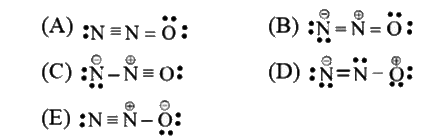
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
CHEMICAL BONDING
MOTION|Exercise EXERCISE -3|18 VideosCHEMICAL BONDING
MOTION|Exercise EXERCISE -4 LEVEL-I|39 VideosCHEMICAL BONDING
MOTION|Exercise EXERCISE -2 LEVEL I|23 VideosCARBOXYLIC ACID
MOTION|Exercise Exercise - 4 (LEVEL - 2 )|22 VideosCHEMICAL EQUILIBRIUM
MOTION|Exercise Exercise - 4|20 Videos
Similar Questions
Explore conceptually related problems
MOTION-CHEMICAL BONDING -EXERCISE -2 LEVEL II
- Which of the following is linear ?
Text Solution
|
- Pick out among the following, species isoelectronic with CO(2).
Text Solution
|
- N2O has a linear, unsymmetrical structure that may be thought of as a...
Text Solution
|
- In which of the following cases the number of lone pairs on central at...
Text Solution
|
- The structure of XeF6 is :
Text Solution
|
- Match the column
Text Solution
|
- Which of the following statement is/are correct
Text Solution
|
- The central atom assume sp^(3) hybridisation in:
Text Solution
|
- Which shows a changes in the type of hybridisation when :
Text Solution
|
- Which of the following statements is/are correct?
Text Solution
|
- Which one of the following compounds has bond angle close to 90^@ ?
Text Solution
|
- The octet rule is not obeyed in :
Text Solution
|
- To which of the following species the octet rule not applicable?
Text Solution
|
- Name the oxide of chlorine which has odd number of electrons and param...
Text Solution
|
- Rotation around the bond (between the underlined atoms) is restricted ...
Text Solution
|
- CO(2) is isostructural with
Text Solution
|
- In the structure of H(2)CSF(4), which of the following statement is/ar...
Text Solution
|
- Which of the following compounds contain(s) both ionic and covalent bo...
Text Solution
|
- Which of the following statements are not correct
Text Solution
|
- Resonance occurs due to the :
Text Solution
|