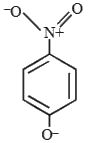
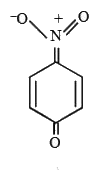
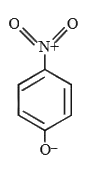
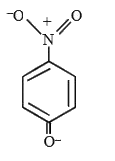
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
CHEMICAL BONDING
MOTION|Exercise EXERCISE -4 LEVEL-I|39 VideosCHEMICAL BONDING
MOTION|Exercise EXERCISE -4 LEVEL-II|40 VideosCHEMICAL BONDING
MOTION|Exercise EXERCISE -2 LEVEL II|71 VideosCARBOXYLIC ACID
MOTION|Exercise Exercise - 4 (LEVEL - 2 )|22 VideosCHEMICAL EQUILIBRIUM
MOTION|Exercise Exercise - 4|20 Videos
Similar Questions
Explore conceptually related problems
MOTION-CHEMICAL BONDING -EXERCISE -3
- The geometry and the type of hybrid orbitals present about the central...
Text Solution
|
- The hybridisation of atomic orbitals of nitrogen in NO2^+ , NO3^- and...
Text Solution
|
- The correct order of hybridisation of the central atom in the oflliwng...
Text Solution
|
- The correct order of increasig C-O bond length of CO, CO(3)^(2-), CO(2...
Text Solution
|
- In the dichromate anion
Text Solution
|
- The geometry of H(2)S and its dipole moment are :
Text Solution
|
- In compounds of type ECI(3), where E=BP, As or B, the angles CI-E-CI f...
Text Solution
|
- The most likely representation of resonance structure of p-nitrophenox...
Text Solution
|
- The common features among the species CN^-, CO and NO^+
Text Solution
|
- Amongst H2O, H2S, H2 Se and H2 Te, the one with the highest boiling po...
Text Solution
|
- Arrange the following species in decreasing order of bond angle. NO2...
Text Solution
|
- Find out the bond order of : (a) H2 (b) H2^(+) (c) He2 (d) Li2 (e) ...
Text Solution
|
- Why does He2^+ exist whereas He2 does not ?
Text Solution
|
- The bond length in O2^+, O2, O2^- and O2^(2-) follows the order :
Text Solution
|
- Write the electronic structures of : (a) CO , (b) NO, (c) HF ,(d) HCl,...
Text Solution
|
- Of the following species which has the shortest bond length NO, NO^+ ,...
Text Solution
|
- Based upon M.O. theory, state reason for the paramagnetic character of...
Text Solution
|
- In the hydrides of group 16th elements the bond angles decrease in the...
Text Solution
|