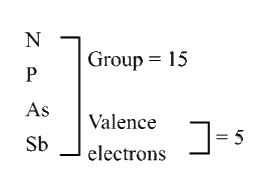
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
MOTION-CHEMICAL BONDING -EXERCISE -4 LEVEL-I
- The structure of IF7 is
Text Solution
|
- Which of the following contains maximum number of pairs around Xe atom...
Text Solution
|
- The number and type of bonds between two carbon atoms in calcium carbi...
Text Solution
|
- In which of the following pairs, the two species are not isostructural...
Text Solution
|
- The molecule having smallest bond angle is
Text Solution
|
- Which one of the following molecules is expected to exhibit diamagneti...
Text Solution
|
- In which of the following paris of molecules/ions, both the species ar...
Text Solution
|
- Stability of the species Li(2), Li(2)^(-) and Li(2)^(+) increases in t...
Text Solution
|
- The correct statement for the molecule CsI(3) is
Text Solution
|
- For which of the following molecule significant mu ne 0 ?
Text Solution
|
- Which one of the following properties is not shown by NO ? .
Text Solution
|
- Which one of the following alkaline earth metal sulphates has its hydr...
Text Solution
|
- The intermolecular interaction that is dependent on the inverse cube o...
Text Solution
|
- Which one has the highest boiling point?
Text Solution
|
- The species in which the N-atom is in a state of sp hybridisation is
Text Solution
|
- The pair in which phosphours atoms have a formed oxidation state of +3...
Text Solution
|
- Which one of the following statements about water is FALSE ?
Text Solution
|
- Which of the following species not paramagnetic ?
Text Solution
|
- The group having isoelectronic species is
Text Solution
|
- Which of the following are Lewis acids?
Text Solution
|