Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
MOTION-CHEMICAL BONDING -EXERCISE -4 LEVEL-II
- Assuming that Hund's rule is violated the bond order and magnetic natu...
Text Solution
|
- All the compounds listed in Column I react with water. Match the resul...
Text Solution
|
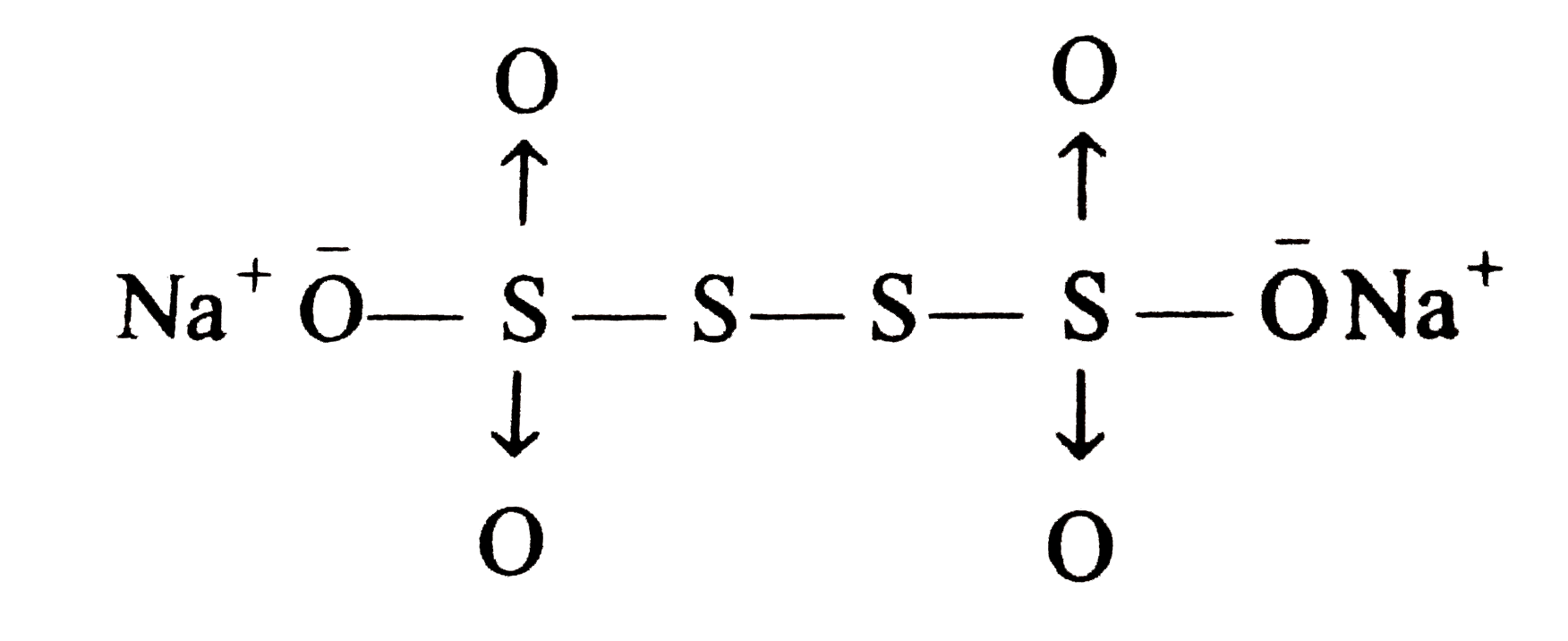
- The difference in the oxidation numbers of the two types of sulphur at...
Text Solution
|
- In allene (C(3)H(4)) the type(s) of hybridisation of the carbon atoms ...
Text Solution
|
- The shape of XeO2F2 molecule is
Text Solution
|
- With respect to graphite and diamond, which of the statements given be...
Text Solution
|
- Which ordering of compounds is according to the decreasing order of th...
Text Solution
|
- The correct statement about O3 is(are)
Text Solution
|
- A list of species having the formula of XZ(4) is given below XeF(4), S...
Text Solution
|
- When O(2) is adsorbed on a metallic surface, electron transfer occurs...
Text Solution
|
- Under hydrolysis conditions, the compounds used for preparation of lin...
Text Solution
|
- The total number of lone pair of electrons in N(2)O(3) is
Text Solution
|
- Among the triatomic molecules/ions BeCl(2),N(3)^(-),N(2)O, NO(2)^(+), ...
Text Solution
|
- The correct statement(s) regarding, (i) HClO, (ii) HClO(2), (iii) HC...
Text Solution
|
- The crystalline form of borax has
Text Solution
|
- The compound(s) with two lone pairs of electron on the central atom is...
Text Solution
|
- MOLECULAR ORBITAL THEORY
Text Solution
|
- The colour of the X(2) molecules of group 17 elements changes graduall...
Text Solution
|
- The sum of the number of lone pairs of electrons on each central atom ...
Text Solution
|
- Among H(2), He(2)^(+), Li(2), Be(2), B(2), C(2), N(2), O(2)^(-) and F(...
Text Solution
|