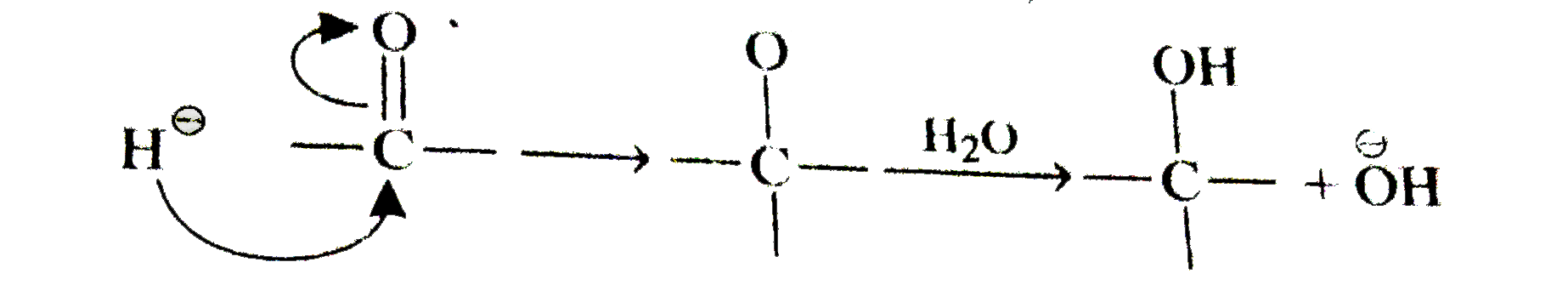
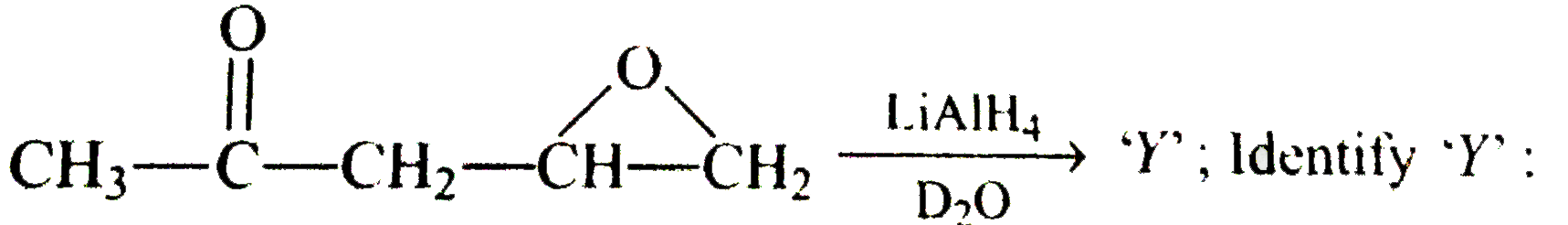
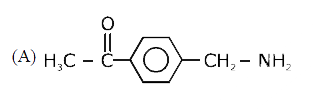
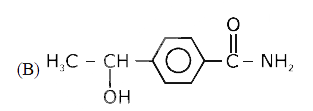
A
B
C
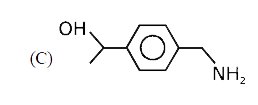
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
ALCOHOLS & ETHERS
MOTION|Exercise EXERCISE -3 OBJECTIVE PROBLEMS JEE ADVANCED|17 VideosALCOHOLS & ETHERS
MOTION|Exercise EXERCISE -4 (LEVEL -I) PREVIOUS YEAR JEE MAIN|12 VideosALCOHOLS & ETHERS
MOTION|Exercise EXERCISE -2 (LEVEL -I) OBJECTIVE PROBLEMS JEE MAIN|38 VideosALKYI HALIDE
MOTION|Exercise Exercise - 4 | Level-II|8 Videos
Similar Questions
Explore conceptually related problems
MOTION-ALCOHOLS & ETHERS-EXERCISE -2 (LEVEL -II) MULTIPLE CORRECT JEE ADVANCED
- Which of the following compound undergo hydrolysis most easily :
Text Solution
|
- Which can be cleaved by HIO4 ?
Text Solution
|
- Explain esterification reaction.
Text Solution
|
- overset(H2//Pd//BaSO4)to (Z) Identify X, Y, Z :
Text Solution
|
- Which is/are corect statement ?
Text Solution
|
- 3-methyl-3-hexanol can be prepared by -
Text Solution
|
- Which compound will be oxidised by HIO(4) ?
Text Solution
|
- Which of the following is/are aromatic ?
Text Solution
|
- Acctaldehyde can be obtained from which of the following rections ?
Text Solution
|
- Find out number of alcohols that can give positive iodoform test.
Text Solution
|
- A tertiary alcohol (H) upon acid-catalysed dehydration gives a product...
Text Solution
|
- A tertiary alcohol (H) upon acid-catalysed dehydration gives a product...
Text Solution
|
- A tertiary alcohol (H) upon acid-catalysed dehydration gives a product...
Text Solution
|
- Carbon oxygen double bond are easily reduced by NaBH(4) or LiAlH(4). T...
Text Solution
|
- Carbon oxygen double bond are easily reduced by NaBH(4) or LiAlH(4). T...
Text Solution
|
- Acid catalysed conversation of 1,2-diol or vicinal, into carbonyl comp...
Text Solution
|
- Acid catalysed conversion of 1,2-diol or vicinal diol, into carbonyl c...
Text Solution
|
- Column - I (A) Oxidation of 1^@ alcohol in aldehyde Oxidation ...
Text Solution
|
- Column- I (A) Identification of 1^@, 2^@ and 3^@ alcohol (B) Ident...
Text Solution
|
- Column - I (A) CH3-overset(O)overset(||)-O-overset(Ph)overset(|)unde...
Text Solution
|