Similar Questions
Explore conceptually related problems
Recommended Questions
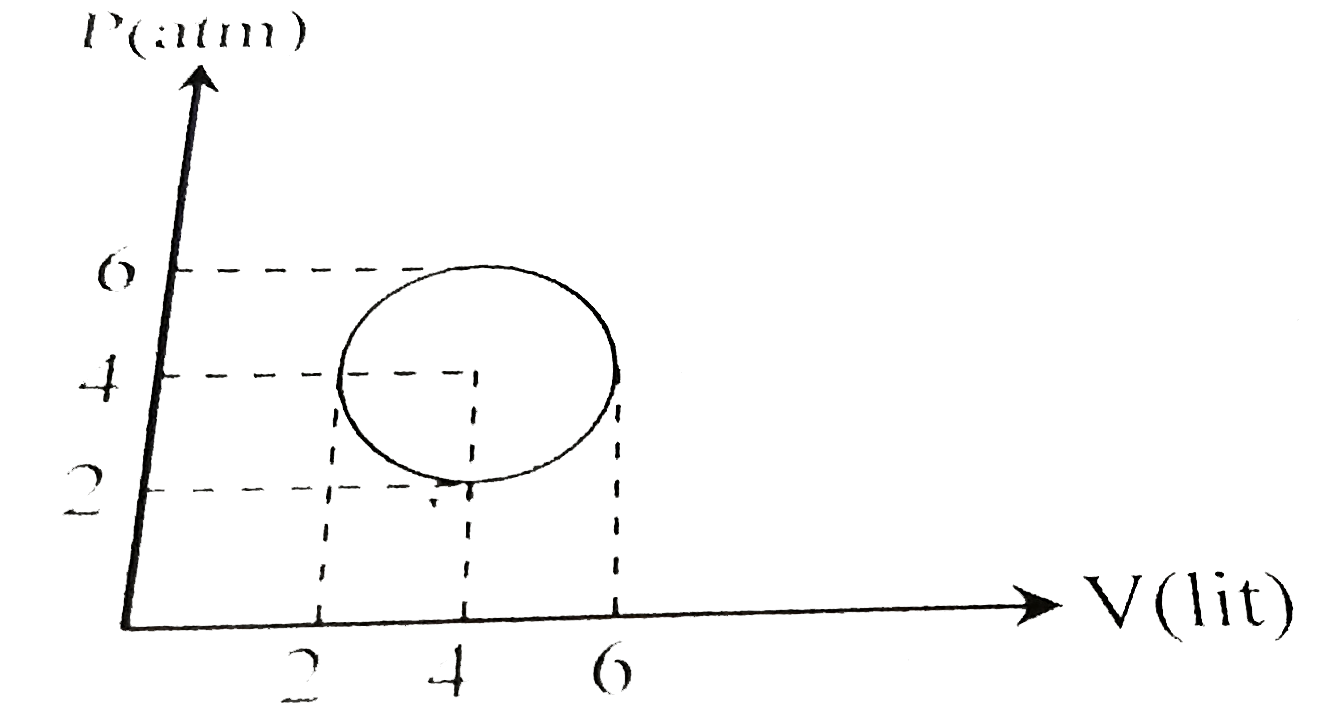
- An ideal gas undergoes a circular cycle centered at 4 atm, 4 lit as sh...
Text Solution
|
- n' moles of an ideal gas undergoes a process AtoB as shown in the figu...
Text Solution
|
- An ideal gas undergoes a circular cycle as shown in the figure. Find t...
Text Solution
|
- An ideal gas undergoes a circular cycle centered at 4 atm, 4 lit as sh...
Text Solution
|
- One mole of an ideal gas is carried through the reversible cyclic proc...
Text Solution
|
- Two moles of an ideal monoatomic gas undergoes a cyclic process ABCA a...
Text Solution
|
- One mole of ideal monatomic gas is carried through the reversible cycl...
Text Solution
|
- Select the correct option for an ideal gas undergoing a process as sho...
Text Solution
|
- One mole of an ideal gas is carried through the reversible cyclic proc...
Text Solution
|