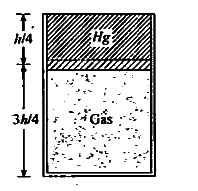
Text Solution
Verified by Experts
Topper's Solved these Questions
Kinetic Theory of Gases and Gas Laws
PHYSICS GALAXY - ASHISH ARORA|Exercise Discussion Question|18 VideosKinetic Theory of Gases and Gas Laws
PHYSICS GALAXY - ASHISH ARORA|Exercise Conceptual MCQs Single Option Correct|27 VideosHEAT TRANSFER
PHYSICS GALAXY - ASHISH ARORA|Exercise Unsolved Numerical Problems for Preparation of NSEP,INPhO&IPhO|66 VideosMAGNETIC EFFECTS OF CURRENT AND MAGNETISM
PHYSICS GALAXY - ASHISH ARORA|Exercise U.N.P|79 Videos
Similar Questions
Explore conceptually related problems
PHYSICS GALAXY - ASHISH ARORA-Kinetic Theory of Gases and Gas Laws-Unsolved Numerical Problems for Preparation of NSEP, INPhO & IPhO
- A tall cylindrical vessel with gaseous nitrogen is located in a unifor...
Text Solution
|
- An electron tube was sealed off during manufacture at a pressure of 1....
Text Solution
|
- The mass of hydrogen molecule is 3.23 xx 10^(-27)Kg. If 10^23 hydrogen...
Text Solution
|
- A lamp of voume 50 cc was sealed off during manufacture at a pressure ...
Text Solution
|
- An electric bulb of volume 250 cm^(3) was sealed off during manufactur...
Text Solution
|
- Calculate the kinetic energy of translation of the molecules of 20g of...
Text Solution
|
- A cubic box of volume 8.0xx10^(-3)m^(3) is filled with air at atmosphe...
Text Solution
|
- Calculate the number of molecules/m^(3) in an ideal gas at STP.
Text Solution
|
- The lowest pressure attainable using the best available vacuum techniq...
Text Solution
|
- In outer space the density of matter is about one atom per cm^(3), mai...
Text Solution
|
- Calculate the density of oxygen at STP using the ideal gas law.
Text Solution
|
- A tank contains 28.0 kg of O(2) gas at a gauge pressure of 6.80 atm. I...
Text Solution
|
- A house has a volume of 600 m^(3). What is the total mass of air ins...
Text Solution
|
- A house has a volume of 600 m^(3). If the temperature rises to 25^(@...
Text Solution
|
- A tire is filled with air at 15^(@)C to a gauge pressure of 1.9xx10^(5...
Text Solution
|
- An electric bulb of volume 250 cm^(3) was sealed off during manufactur...
Text Solution
|
- A column of mercury of 10cm length is contained in the middle of a nar...
Text Solution
|
- Two cylinder having m(1)g and m(2)g of a gas at pressure P(1) and P(2...
Text Solution
|
- In a toy truck the volume of its tyre tube is 2000 cm^(3) in which air...
Text Solution
|
- Show that the volume thermal expansion coefficient for an ideal gas at...
Text Solution
|
- The temperature of a room of volume Vrise from T(1) to T(2). How much ...
Text Solution
|