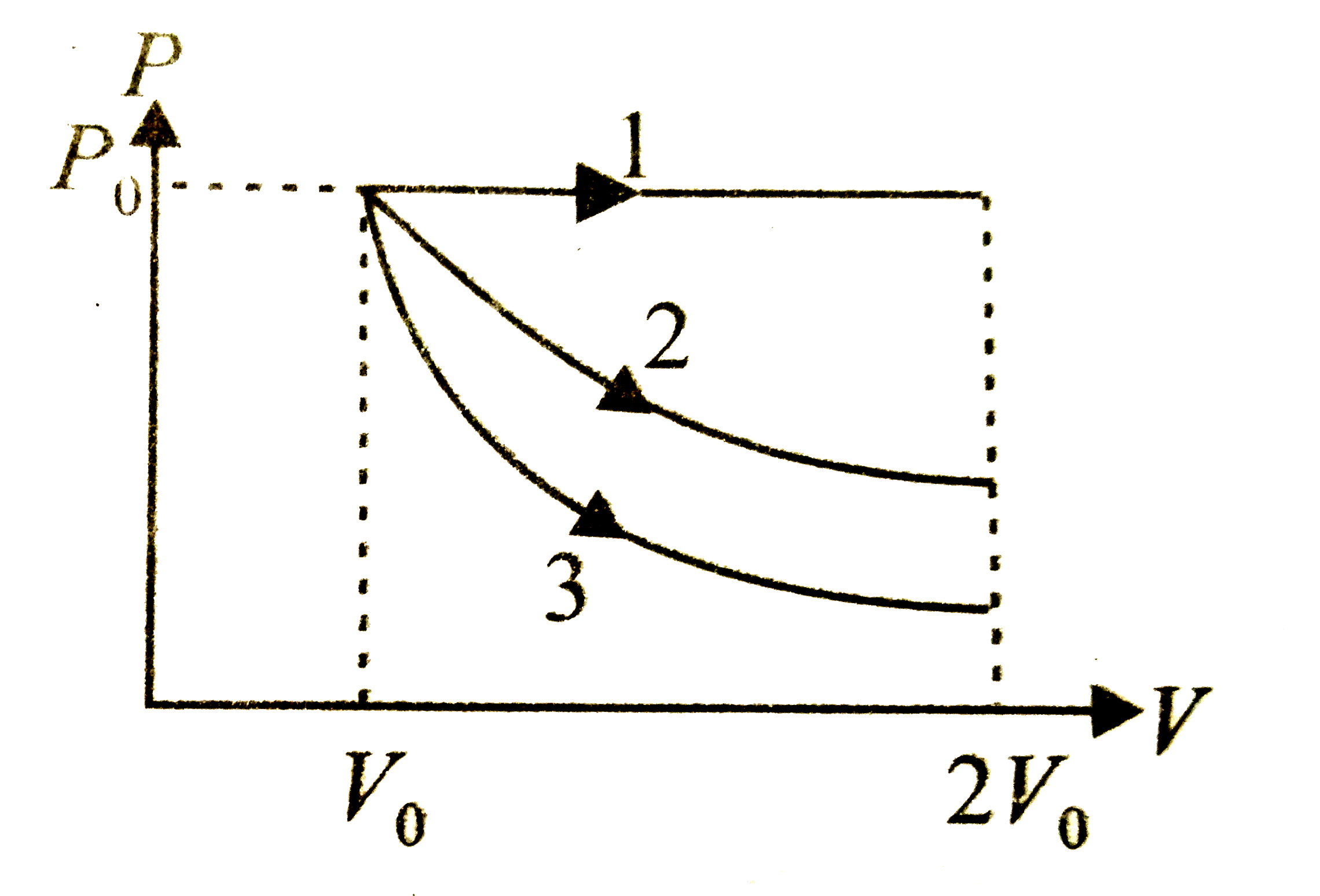
A
B
C
D
Text Solution
Verified by Experts
Topper's Solved these Questions
Kinetic Theory of Gases and Gas Laws
PHYSICS GALAXY - ASHISH ARORA|Exercise Numerical MCQs Single Options Correct|28 VideosKinetic Theory of Gases and Gas Laws
PHYSICS GALAXY - ASHISH ARORA|Exercise Advance MCQs with One or More Options Correct|15 VideosKinetic Theory of Gases and Gas Laws
PHYSICS GALAXY - ASHISH ARORA|Exercise Discussion Question|18 VideosHEAT TRANSFER
PHYSICS GALAXY - ASHISH ARORA|Exercise Unsolved Numerical Problems for Preparation of NSEP,INPhO&IPhO|66 VideosMAGNETIC EFFECTS OF CURRENT AND MAGNETISM
PHYSICS GALAXY - ASHISH ARORA|Exercise U.N.P|79 Videos
Similar Questions
Explore conceptually related problems
PHYSICS GALAXY - ASHISH ARORA-Kinetic Theory of Gases and Gas Laws-Conceptual MCQs Single Option Correct
- When an ideal gas is compressed isothermally then its pressure increas...
Text Solution
|
- Three closed vessels A, B and C are at the same temperature T and cont...
Text Solution
|
- The pressure p of a gas is plotted against its absolute temperature T ...
Text Solution
|
- Pressure versus temperature graph of an ideal gas of equal number of m...
Text Solution
|
- A gas is contained in a metallic cylinder fitted with a piston.The pis...
Text Solution
|
- The figure shows two paths for the change of state of a gas from A to ...
Text Solution
|
- A graph is plotted with PV/T on y-axis and mass of the gas along x-axi...
Text Solution
|
- A volume V of air saturated with water vapour experts a pressure P. Pr...
Text Solution
|
- Two samples A and B are initially kept in the same state. The sample A...
Text Solution
|
- A gas has molar heat capacity C=24.9 J mol^(-1)'K^(-1) in the process ...
Text Solution
|
- A horizontal cylinder has two sections of unequal cross - sections, in...
Text Solution
|
- Pressure versus temperature graph of an ideal gas are as shown in figu...
Text Solution
|
- Pressure versus temperature graph of an ideal gas is shown in figure. ...
Text Solution
|
- The quantity (pV)/(kt) represents
Text Solution
|
- The equation of state for a real gas such as hydrogen, oxygen, etc. is...
Text Solution
|
- A stationary vertical cylindrical container of very large height fille...
Text Solution
|
- The coefficient of linear expansion of an in homogeneous rod change li...
Text Solution
|
- A gas is expanded form volume V(0) to 2V(0) under three different pro...
Text Solution
|
- Some of the thermodynamic parameters are state variables while some ar...
Text Solution
|
- P-T graphs of an ideal gas are as shown in figures-2.42 below. Choose ...
Text Solution
|