Similar Questions
Explore conceptually related problems
Recommended Questions
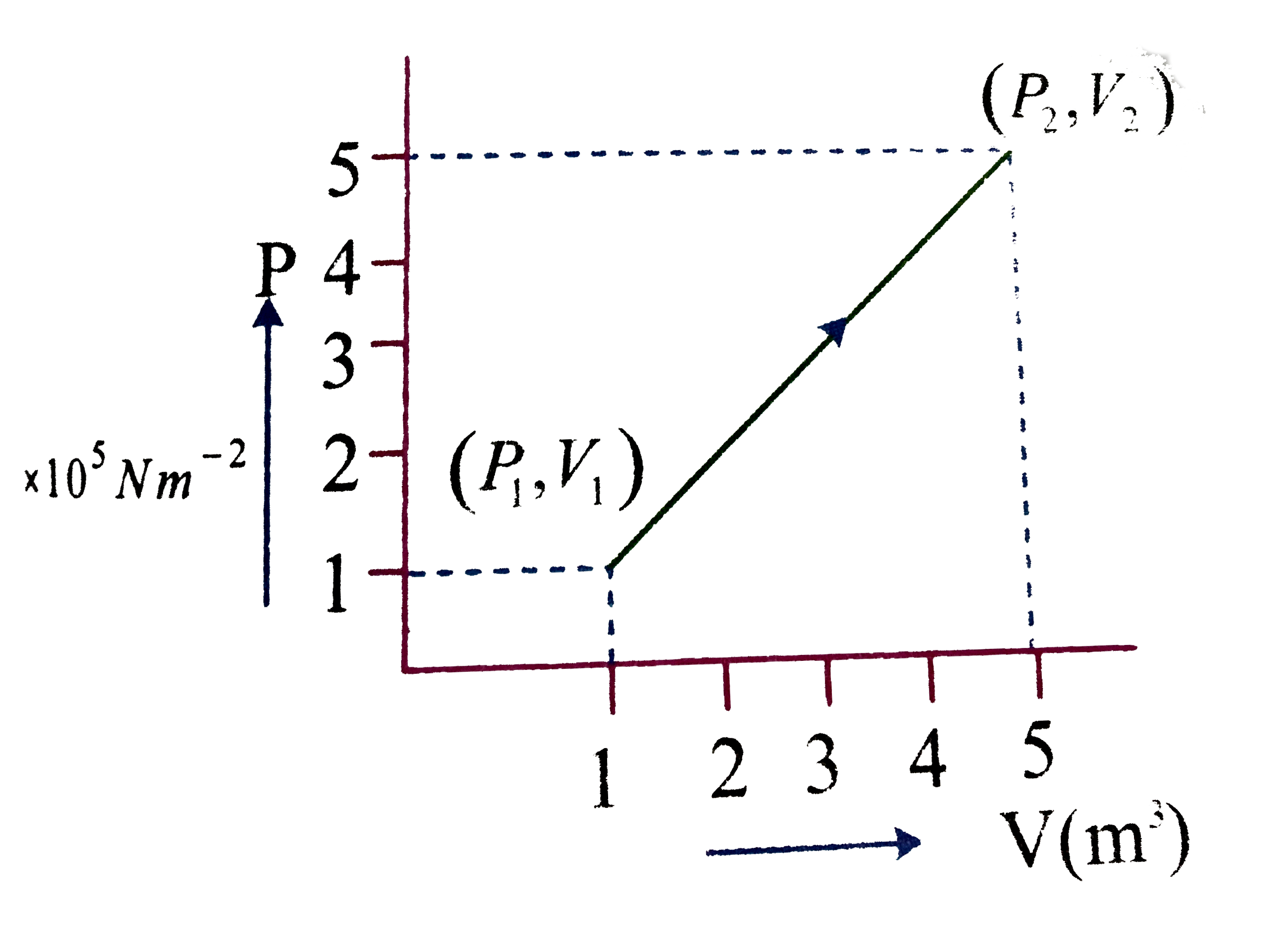
- A system changes from the state (P(1),V(1)) to (P(2)V(2)) as shwon in ...
Text Solution
|
- A system can be taken from the initial state p(1),V(1) to the final st...
Text Solution
|
- A system changes from the state (P(1),V(1)) to (P(2)V(2)) as shwon in ...
Text Solution
|
- A thermo-dynamical system is changed from state ) ,(P(1),V(1)) to (P(2...
Text Solution
|
- A gaseous system changes from state A(P(1), V(1), T(1)) to B(P(2), V(2...
Text Solution
|
- A gaseous system changes from state A(P(1),V(1),T(1)) to B(P(2),V(2),T...
Text Solution
|
- A gaseous system changes from state A (P(1), V(1),T(1)) to B (P(2), V(...
Text Solution
|
- यदि रुद्धोष्म परिवर्तन में गैस की अवस्था P(1),V(1),T(1) सेP(2),V(2),T(...
Text Solution
|
- A system changes from the state (P(1), V(1)) to (P(2) , V(2)) as shown...
Text Solution
|