A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- Ionization energies of five elements in kcal/mol are given below: . ...
Text Solution
|
- If Q reacts with fluorine and oxygen, the molecular formula of fluorid...
Text Solution
|
- Ionization energies of five elements in kcal/mol are given below: Q. T...
Text Solution
|
- Ionization energies of five elements in kcal/mol are given below: ...
Text Solution
|
- Ionization energies of five elements in kcal/mol are given below: . ...
Text Solution
|
- Ionization energies of five elements in kcal/mol are given below: . If...
Text Solution
|
- Ionization energies of five elements in kcal/mol are given below: ...
Text Solution
|
- Ionization energies of five elements in kcal/mol are given below: Q. T...
Text Solution
|
- Ionization energies of five elements in kcal/mol are given below: Q. I...
Text Solution
|
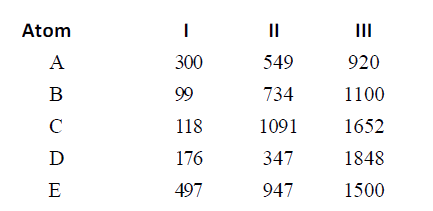 .
.