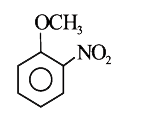
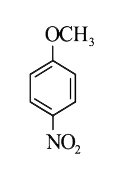
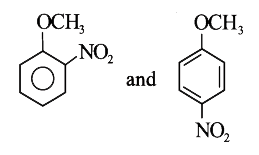
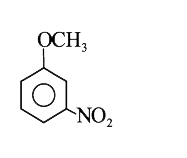
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- Methyl phenyl ether reaction with HNO3 and H2SO4 results .
Text Solution
|
- The heating of phenyl-methyl ethers with HI produces
Text Solution
|
- Describe the action of hydroiodic acid on methyl phenyl ether.
Text Solution
|
- Methyl phenyl ether can be obtained by reacting
Text Solution
|
- The heating of phenyl-methyl ethers with HI produces .
Text Solution
|
- Which reagent is used for bromination of methyl phenyl ether ?
Text Solution
|
- HI के साथ फिनायल मिथायल ईथर गर्म करने पर देता है-
Text Solution
|
- Methyl phenyl ether reaction with HNO3 and H2SO4 results .
Text Solution
|
- फेनिल मेथिल ईथर को HI के साथ गर्म करने पर बनता है
Text Solution
|