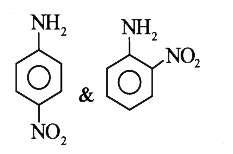
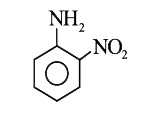
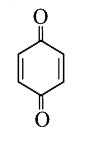
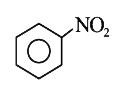
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- Aniline when treated with K2 Cr2 O7 in conc H2 SO4 gives :
Text Solution
|
- From the following information X + H2 SO4 rarr Y (a colourless and i...
Text Solution
|
- In the chemical reaction, K2 CR2 O7 + xH2 SO4 + ySO2 rarr K2SO4 + Cr2(...
Text Solution
|
- When K2 Cr2 O7 crystals are heated with conc. HCl, the gas evolved is
Text Solution
|
- The reaction of K2 Cr2 O7 with NaCl and conc. H2 SO4 gives.
Text Solution
|
- Aniline when treated with K2 Cr2 O7 in conc H2 SO4 gives :
Text Solution
|
- Alcohol on refluxing with K2 Cr2 O7 gives .....
Text Solution
|
- Complete the following reactions : (a) H2 S+ H2 O2 to (b) H2 O2 + 2OH^...
Text Solution
|
- প্রদত্ত কোন্ বক্তব্যটি (গুলি) সঠিক, যখন NaCl ও K2 Cr2 O7 -এর একটি মিশ্...
Text Solution
|