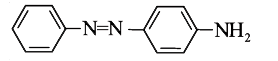
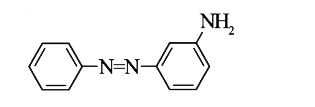
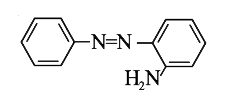A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- When aniline is treated with benzene diazonium chloride at low ...
Text Solution
|
- Benzene diazonium chloride is the product when aniline is treated with
Text Solution
|
- When benzene diazonium chloride is treated with cuprous chloride and H...
Text Solution
|
- Benzene diazonium chloride on reaction with phenol in weakly basic med...
Text Solution
|
- When aniline is treated with benzene diazonium chloride at low ...
Text Solution
|
- Benzene diazonium chloride on reaction with phenol in weakly basic med...
Text Solution
|
- Benzene diazonium chloride on reaction with phenol in weakly basic med...
Text Solution
|
- Benzene diazonium chloride on reaction with phenol in weakly basic med...
Text Solution
|
- When aniline is treated with benzene diazonium chloride at low tempera...
Text Solution
|