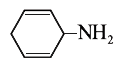
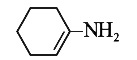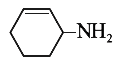A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- The structurtal formula of the compound A, (C6 ,H(11) N) which...
Text Solution
|
- Which one of the following is an optically acitve compound?
Text Solution
|
- An optically active compound with molecular formula C6 H(11) N dissolv...
Text Solution
|
- The structural formula of a compound, C(6)H(11) N (X) that is opticall...
Text Solution
|
- The compound X (C(4)H(11)N) on treatement with nitrous acid gives a te...
Text Solution
|
- Compound A is chiral and has the molecular formula C(8)H(11)N. When A ...
Text Solution
|
- An optically active compound [A] C(5)H(13)N reacts with alkaline CHCl(...
Text Solution
|
- Suggest a structural formula of a compound having molecular C(6)...
Text Solution
|
- Optical active amine of molecular formula C(4)H(11)N on reaction wi...
Text Solution
|