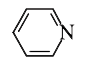
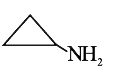
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- Which of the following species contains 5 H atoms , sp ^2 hybr...
Text Solution
|
- The species in which the N-atom is in a state of sp hybridisation is
Text Solution
|
- The species in which the N atom is in a state of sp hybridisation is
Text Solution
|
- In which of the following N atom is not sp^(2) hybridised?
Text Solution
|
- Which of the following species contains 5 H atoms , sp ^2 hybridised N...
Text Solution
|
- In which of the following species, sulphur atom undergoes sp^(3)d hybr...
Text Solution
|
- Which possesses tetrahedral shape (sp^(3)- hybridisation of central at...
Text Solution
|
- Compound containing sp and sp^(2) hybridised carbon atoms is
Text Solution
|
- The species in which the N-atom is in a state of sp hybridisation is
Text Solution
|