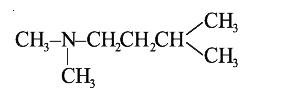A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- Which of the following is not the metamer of CH3CH2-underset(CH3)under...
Text Solution
|
- यौगिक के (IUPAC) नाम लिखिए : CH3CH2-O-underset(CH3)underset(|)(CH)-C...
Text Solution
|
- CH3- underset( OH) underset(|) CH-CH3 का सही नाम निम्नलिखित में कौन ह...
Text Solution
|
- The order of reactivity of following alcohols with halogen acids is . ...
Text Solution
|
- Which of the following is not the metamer of CH3CH2-underset(CH3)under...
Text Solution
|
- CH3-underset(CH3)underset(|)N-underset(C2H5)underset(|)overset(CH3)ove...
Text Solution
|
- CH3CH2-overset(OH)overset(|)underset(Ph)underset(|)C-CH3 निम्न में से ...
Text Solution
|
- Which of the following tertiary (3^@) Is oriented (a) CH3-underset(CH3...
Text Solution
|
- The order of reactivity of following alcohols with halogen acids is . ...
Text Solution
|