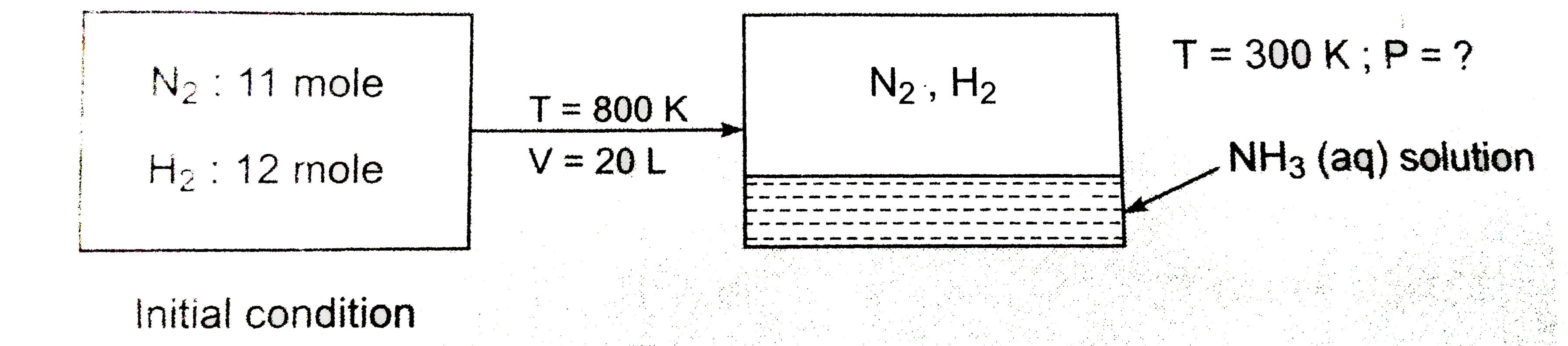11 moles of `N_(2)` and 12 moles of `H_(2)` mixture reacted in 2.0 litre vessel at 800 K. After equilibrium was reached, 6 mole of `H_(2)` was present. 3.58 litre of liquid water is injected in equibrium mixture and resultant gaseous mixture suddenly cooled to 300K. What is the final pressure of gaseous mixture? Neglect vapour pressure of liquid solution. Assume (i) all `NH_(3)` dissolved in water (ii) no change in volume of liquid (iii) At 300 K no reaction takes place between `N_(2)` and `H_(2)`