Three closed rigid vessels, A, B and C, which initially contain three different gases at different temperatures are connected by tube of negligible volume, without any energy transfer with surroundings. The vessel A contain 2 mole Ne gas, at 300 K, vessel 'B' contain 2 mole `SO_(2)` gas at 400 K and vessel 'C' contain 3 mole `CO_(2)` gas at temperature 500 K. What is the final pressure (in atm) attained by gases when all valves of connecting three vessels are opened and additional 15.6 kcal hear supplied to vessels through valve. The volume of A, B and C vessel is 2, 2 and 3 litre respectively
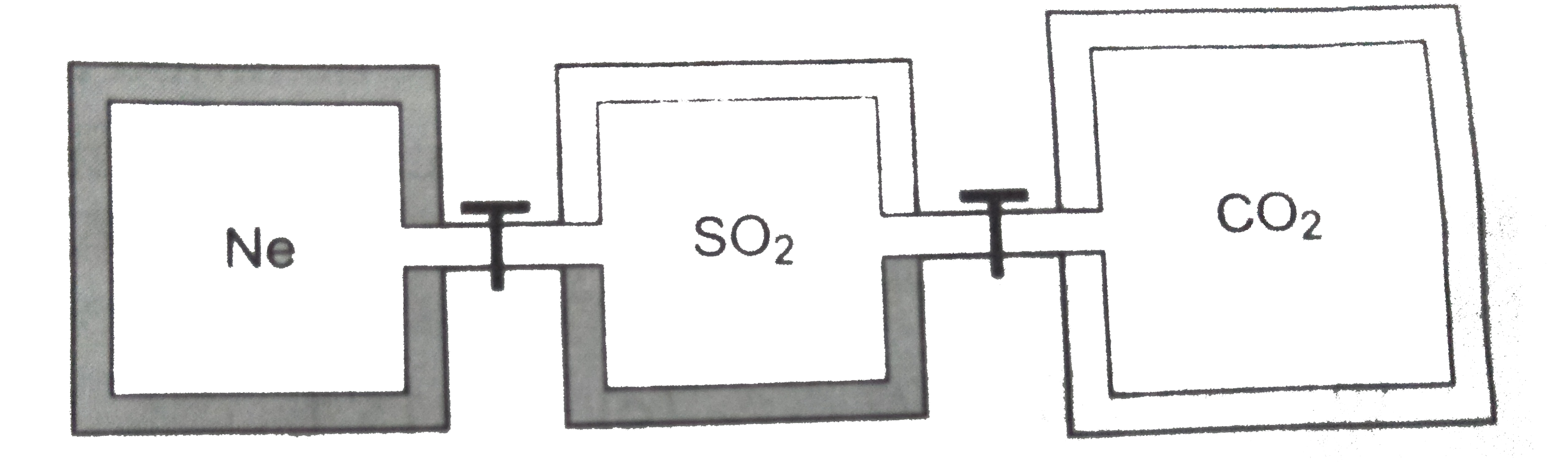
Given :R =2 calorie/mol-K,`C_(v)(Ne)=3//2R,C_(v)(CO)=5//2R and C_(v)(SO_(2))=3R`