At room temperature following traction goes to completion
`2AB(g)+B_(2)(g)rarr2AB_(2)(s)`
`AB_(2)` is solid with negligble vapour pressure below `0^(@)C`. At 300 K, the AB in the smaller flask exerts a pressure of 3 atm and in the larger flask `B_(2)` exerts a pressure of 1 atm at 400 K when they are separated out by a close valve, The gases are mixed by opening the stop cock and after the end of the reaction the flask are cooled to 250 K
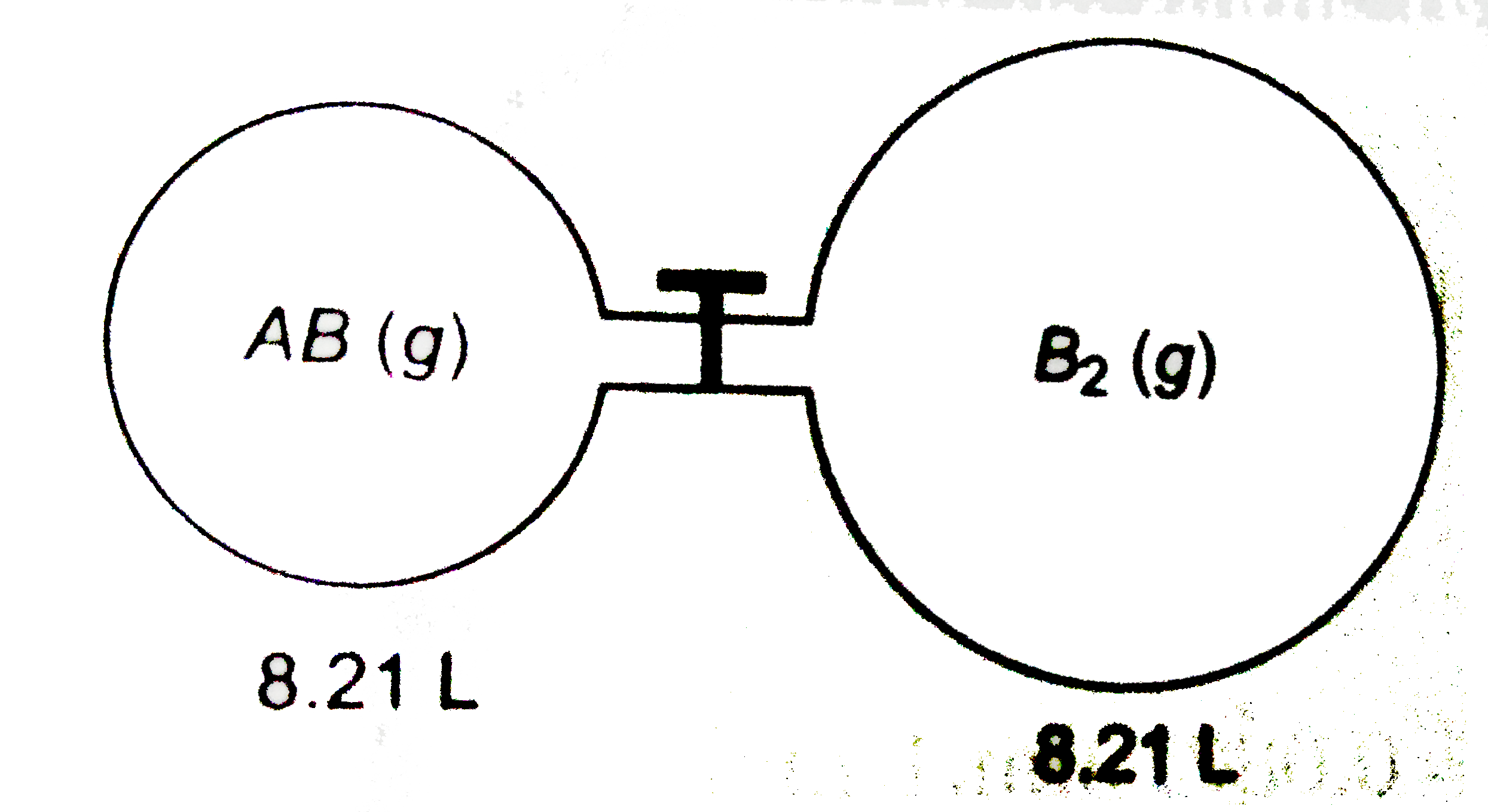
The final pressure is :