Text Solution
Verified by Experts
Topper's Solved these Questions
ATOMIC STRUCTURE
VGS PUBLICATION-BRILLIANT|Exercise LONG ANSWER QUESTIONS|15 VideosATOMIC STRUCTURE
VGS PUBLICATION-BRILLIANT|Exercise ADDITIONAL QUESTIONS & ANSWERS|22 VideosATOMIC STRUCTURE
VGS PUBLICATION-BRILLIANT|Exercise ADDITIONAL QUESTIONS & ANSWERS|22 VideosAP MARCH-2018 I.P.E. PAPER
VGS PUBLICATION-BRILLIANT|Exercise SECTION-C|5 VideosCHEMICAL BONDING AND MOLECULAR STRUCTURE
VGS PUBLICATION-BRILLIANT|Exercise LONG ANSWER QUESTIONS|9 Videos
Similar Questions
Explore conceptually related problems
VGS PUBLICATION-BRILLIANT-ATOMIC STRUCTURE-SHORT ANSWER QUESTIONS
- What is the wavelength of light emitted when the electron in a hydrog...
Text Solution
|
- An atom of an element contains 29 electrons ans 35 neutrons . Deduce (...
Text Solution
|
- Explain giving reasons , which of the following sets of quantum number...
Text Solution
|
- Show that the circumfernce of the Bohr orbit for the hydrogen atom is ...
Text Solution
|
- The longest wavelength doublet absorption transition is observed at 58...
Text Solution
|
- What are the main features of quantum mechanical model of an atom?
Text Solution
|
- What is a nodal plane ? How many nodal plance are possible for 2p and ...
Text Solution
|
- The Lyman series occurs between 91.2 nm and 121.6 nm , the Balmer seri...
Text Solution
|
- How are the quantum numbers n , 1 m(1) for hydrogen atom are obtained...
Text Solution
|
- A line in Lyman series of hydrogen atom has a wavelength of 1.03xx10^(...
Text Solution
|
- If the position of the electon is meaured within an accuracy of pm0.00...
Text Solution
|
- If the velocity of the electron is 1.6xx10^(6) "ms"^(-1) , calculate t...
Text Solution
|
- Explain the difference between emission and absorpation spectra.
Text Solution
|
- The quantum numbers of electrons are given below. Arrange them in orde...
Text Solution
|
- The work function for Cesium atom is 1.9 eV . Calculate the threshold ...
Text Solution
|
- Calculate the wavelength for the emission transition if it starts from...
Text Solution
|
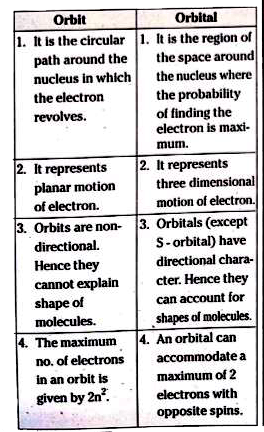
- What is the difference between an orbit and orbital ?
Text Solution
|
- Explain the photoelectric effect .
Text Solution
|